The Anti-Virulence Activities of the Antihypertensive Drug Propranolol in Light of Its Anti-Quorum Sensing Effects against Pseudomonas aeruginosa and Serratia marcescens
- PMID: 38137382
- PMCID: PMC10741015
- DOI: 10.3390/biomedicines11123161
The Anti-Virulence Activities of the Antihypertensive Drug Propranolol in Light of Its Anti-Quorum Sensing Effects against Pseudomonas aeruginosa and Serratia marcescens
Abstract
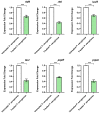
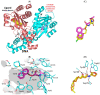
The development of bacterial resistance is an increasing global concern that requires discovering new antibacterial agents and strategies. Bacterial quorum sensing (QS) systems play important roles in controlling bacterial virulence, and their targeting could lead to diminishing bacterial pathogenesis. In this context, targeting QS systems without significant influence on bacterial growth is assumed as a promising strategy to overcome resistance development. This study aimed at evaluating the anti-QS and anti-virulence activities of the β-adrenoreceptor antagonist propranolol at sub-minimal inhibitory concentrations (sub-MIC) against two Gram-negative bacterial models Pseudomonas aeruginosa and Serratia marcescens. The effect of propranolol on the expression of QS-encoding genes was evaluated. Additionally, the affinity of propranolol to QS receptors was virtually attested. The influence of propranolol at sub-MIC on biofilm formation, motility, and production of virulent factors was conducted. The outcomes of the propranolol combination with different antibiotics were assessed. Finally, the in vivo protection assay in mice was performed to assess propranolol's effect on lessening the bacterial pathogenesis. The current findings emphasized the significant ability of propranolol at sub-MIC to reduce the formation of biofilms, motility, and production of virulence factors. In addition, propranolol at sub-MIC decreased the capacity of tested bacteria to induce pathogenesis in mice. Furthermore, propranolol significantly downregulated the QS-encoding genes and showed significant affinity to QS receptors. Finally, propranolol at sub-MIC synergistically decreased the MICs of different antibiotics against tested bacteria. In conclusion, propranolol might serve as a plausible adjuvant therapy with antibiotics for the treatment of serious bacterial infections after further pharmacological and pharmaceutical studies.
Keywords: Pseudomonas aeruginosa; Serratia marcescens; bacterial virulence; drug repurposing; propranolol; quorum sensing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

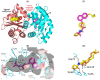


Similar articles
-
Antimicrobial and anti-virulence activities of 4-shogaol from grains of paradise against gram-negative bacteria: Integration of experimental and computational methods.J Ethnopharmacol. 2024 Apr 6;323:117611. doi: 10.1016/j.jep.2023.117611. Epub 2023 Dec 27. J Ethnopharmacol. 2024. PMID: 38158095
-
Characterization of the Anti-Biofilm and Anti-Quorum Sensing Activities of the β-Adrenoreceptor Antagonist Atenolol against Gram-Negative Bacterial Pathogens.Int J Mol Sci. 2022 Oct 28;23(21):13088. doi: 10.3390/ijms232113088. Int J Mol Sci. 2022. PMID: 36361877 Free PMC article.
-
Muting Bacterial Communication: Evaluation of Prazosin Anti-Quorum Sensing Activities against Gram-Negative Bacteria Pseudomonas aeruginosa, Proteus mirabilis, and Serratia marcescens.Biology (Basel). 2022 Sep 13;11(9):1349. doi: 10.3390/biology11091349. Biology (Basel). 2022. PMID: 36138828 Free PMC article.
-
Sesamin and sesamolin rescues Caenorhabditis elegans from Pseudomonas aeruginosa infection through the attenuation of quorum sensing regulated virulence factors.Microb Pathog. 2021 Jun;155:104912. doi: 10.1016/j.micpath.2021.104912. Epub 2021 Apr 28. Microb Pathog. 2021. PMID: 33932548 Review.
-
Quorum Sensing Inhibitors to Quench P. aeruginosa Pathogenicity.Pharmaceuticals (Basel). 2021 Dec 5;14(12):1262. doi: 10.3390/ph14121262. Pharmaceuticals (Basel). 2021. PMID: 34959667 Free PMC article. Review.
Cited by
-
Sulforaphane Is Not Only a Food Supplement: It Diminishes the Intracellular Survival and Colonization of Salmonella enterica.ACS Omega. 2025 Jan 16;10(3):2969-2977. doi: 10.1021/acsomega.4c09408. eCollection 2025 Jan 28. ACS Omega. 2025. PMID: 39895767 Free PMC article.
-
Reviving Furosemide as a Metallo-β-Lactamase Inhibitor against MDR Acinetobacter baumannii.J Microbiol Biotechnol. 2025 Aug 7;35:e2506023. doi: 10.4014/jmb.2506.06023. J Microbiol Biotechnol. 2025. PMID: 40774820 Free PMC article.
-
Cilostazol is a promising anti-pseudomonal virulence drug by disruption of quorum sensing.AMB Express. 2024 Aug 1;14(1):87. doi: 10.1186/s13568-024-01740-1. AMB Express. 2024. PMID: 39090255 Free PMC article.
-
Redirecting pantoprazole as a metallo-beta-lactamase inhibitor in carbapenem-resistant Klebsiella pneumoniae.Front Pharmacol. 2024 Mar 12;15:1366459. doi: 10.3389/fphar.2024.1366459. eCollection 2024. Front Pharmacol. 2024. PMID: 38533260 Free PMC article.
-
Nanocomposite alginate hydrogel loaded with propranolol hydrochloride kolliphor® based cerosomes as a repurposed platform for Methicillin-Resistant Staphylococcus aureus-(MRSA)-induced skin infection; in-vitro, ex-vivo, in-silico, and in-vivo evaluation.Drug Deliv Transl Res. 2025 Feb;15(2):556-576. doi: 10.1007/s13346-024-01611-z. Epub 2024 May 18. Drug Deliv Transl Res. 2025. PMID: 38762697 Free PMC article.
References
-
- Al-Majed A.A., Bakheit A.H., Aziz H.A.A., Alajmi F.M., AlRabiah H. Profiles of Drug Substances, Excipients and Related Methodology. Volume 42. Elsevier; Amsterdam, The Netherlands: 2017. Propranolol; pp. 287–338. - PubMed
-
- Chaikof E.L., Dalman R.L., Eskandari M.K., Jackson B.M., Lee W.A., Mansour M.A., Mastracci T.M., Mell M., Murad M.H., Nguyen L.L. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 2018;67:2–77.e72. doi: 10.1016/j.jvs.2017.10.044. - DOI - PubMed
LinkOut - more resources
Full Text Sources

