Constitutive HO-1 and CD55 (DAF) Expression and Regulatory Interaction in Cultured Podocytes
- PMID: 38137516
- PMCID: PMC10740928
- DOI: 10.3390/biomedicines11123297
Constitutive HO-1 and CD55 (DAF) Expression and Regulatory Interaction in Cultured Podocytes
Abstract
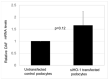
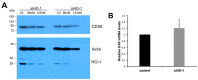
Overexpression of the inducible heme oxygenase (HO-1) isoform in visceral renal glomerular epithelial cells (podocytes) using in vivo transgenesis methods was shown to increase glomerular expression of the complement regulatory protein decay-accelerating factor (DAF, CD55) and reduce complement activation/deposition in a rat model of immune-mediated injury. In this preliminary study, we assessed whether constitutively expressed HO-1 regulates CD55 expression in cultured rat podocytes. We employed methods of flow cytometry, quantitative (q) RT-qPCR and post-transcriptional HO-1 gene silencing (HO-1 interfering RNA, RNAi), to assess changes in constitutive (basal) levels of podocyte HO-1 and CD55 mRNA in cultured rat podocytes. Additionally, the effect of the HO-1 inducer, heme, on HO-1 and CD55 expression was assessed. Results indicate that rat podocytes constitutively express HO-1 and DAF and that the HO-1 inducer, heme, increases both HO-1 and DAF expression. HO-1 gene silencing using RNA interference (RNAi) is feasible but the effect on constitutive CD55 transcription is inconsistent. These observations are relevant to conditions of podocyte exposure to heme that can activate the complementary cascade, as may occur in systemic or intraglomerular hemolysis.
Keywords: CD55; DAF; HO-1; hemin; podocytes; siRNA.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





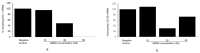

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

