Early Initiation of Adalimumab Significantly Diminishes Postoperative Crohn's Disease Endoscopic Recurrence and Is Superior to 6-Mercaptopurine Therapy: An Open-Label, Randomized Controlled Study
- PMID: 38137669
- PMCID: PMC10743980
- DOI: 10.3390/jcm12247600
Early Initiation of Adalimumab Significantly Diminishes Postoperative Crohn's Disease Endoscopic Recurrence and Is Superior to 6-Mercaptopurine Therapy: An Open-Label, Randomized Controlled Study
Abstract
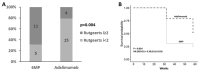
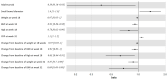
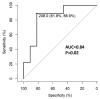
Postoperative recurrence (POR) is the rule in patients with Crohn's disease (CD), mitigated with prophylactic therapy. The evidence for therapeutic choice and timing of intervention is lacking. We aimed to compare the rates of POR in patients treated early with prophylactic 6-mercaptopurine (6-MP) or adalimumab. We conducted a prospective single-center randomized open-label clinical study in which patients in surgical remission following their first ileocecectomy were randomized to receive early treatment with 6-MP or adalimumab. Patients were followed up clinically every 3 months and underwent endoscopy at weeks 32 and 58 postoperatively. The primary endpoint was endoscopic recurrence (ePOR) at 1 year (week 58), defined as a Rutgeerts score ≥ i2. We enrolled 35 patients (25 males, mean age 35 ± 1.4 years, median disease duration 5 ± 6.1 years) following ileocecectomy. Of these, seven (20%) were current smokers and nine (26%) biologics-experienced. Patients allocated to adalimumab had significantly less ePOR than patients treated with 6MP at week 32 (21% vs. 69%, p = 0.004) and 58 (47% vs. 75%), (p = 0.03, HR = 0.39, 95% CI = 0.16-0.93). POR was associated with an increased diameter of the resected small bowel surgical specimen, lower baseline body mass index (BMI), increased week 18 fecal calprotectin, increased week 18 serum alanine aminotransferase and decreased week 18 hemoglobin level. Adalimumab was more effective than 6-MP in preventing ePOR. Increased operative small bowel diameter and lower postoperative BMI were associated with ePOR. At eighteen weeks, serum hemoglobin, ALT and fecal calprotectin levels were predictive of endoscopic disease recurrence. (ClinicalTrials.gov ID NCT01629628).
Keywords: 6-mercaptopurine; Crohn; adalimumab; clinical trial; surgery.
Conflict of interest statement
The authors received previously honoraria, speaking fees and educational and research grants from ABBVIE, the manufacturer of Humira and funder of this trial. Ayal Hirsch: honoraria, speaking fees and educational grant. Hagit Tulchinsky: speaking fees. Henit Yanai: Honoraria, speaking fees, consulting fees and participation in a data safety monitoring board or advisory board. Iris Dotan: honoraria, speaking fees and educational and research grants. Nitsan Maharshak: honoraria, speaking fees and educational and research grants. The funders had no role in the design of this study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Adalimumab for maintenance of remission in Crohn's disease.Cochrane Database Syst Rev. 2020 May 16;5(5):CD012877. doi: 10.1002/14651858.CD012877.pub2. Cochrane Database Syst Rev. 2020. PMID: 32413933 Free PMC article.
-
Effect of tight control management on Crohn's disease (CALM): a multicentre, randomised, controlled phase 3 trial.Lancet. 2017 Dec 23;390(10114):2779-2789. doi: 10.1016/S0140-6736(17)32641-7. Epub 2017 Oct 31. Lancet. 2017. PMID: 29096949 Clinical Trial.
-
Intensive drug therapy versus standard drug therapy for symptomatic intestinal Crohn's disease strictures (STRIDENT): an open-label, single-centre, randomised controlled trial.Lancet Gastroenterol Hepatol. 2022 Apr;7(4):318-331. doi: 10.1016/S2468-1253(21)00393-9. Epub 2021 Dec 8. Lancet Gastroenterol Hepatol. 2022. PMID: 34890567 Clinical Trial.
-
Adalimumab in prevention of postoperative recurrence of Crohn's disease in high-risk patients.World J Gastroenterol. 2012 Aug 28;18(32):4391-8. doi: 10.3748/wjg.v18.i32.4391. World J Gastroenterol. 2012. PMID: 22969204 Free PMC article.
-
Colonoscopy-guided therapy for the prevention of post-operative recurrence of Crohn's disease.Cochrane Database Syst Rev. 2020 Aug 3;8(8):CD012328. doi: 10.1002/14651858.CD012328.pub2. Cochrane Database Syst Rev. 2020. PMID: 32746500 Free PMC article.
Cited by
-
Preventing Recurrence of Crohn's Disease Post-Ileocaecal Surgery in Paediatric Patients: A Therapy Guide Based on Systematic Review of the Evidence.Paediatr Drugs. 2024 Nov;26(6):659-672. doi: 10.1007/s40272-024-00650-w. Epub 2024 Aug 31. Paediatr Drugs. 2024. PMID: 39215954
-
Predictive value of long non-coding RNA DDX11-AS1 in inflammatory bowel disease and its effect on intestinal mucosal cell function.Cent Eur J Immunol. 2025;50(1):87-97. doi: 10.5114/ceji.2025.149579. Epub 2025 Apr 16. Cent Eur J Immunol. 2025. PMID: 40620651 Free PMC article.
References
-
- Szanto K., Nyári T., Balint A., Bor R., Milassin Á., Rutka M., Fábián A., Szepes Z., Nagy F., Molnar T., et al. Biological therapy and surgery rates in inflammatory bowel diseases—Data analysis of almost 1000 patients from a Hungarian tertiary IBD center. PLoS ONE. 2018;13:e0200824. doi: 10.1371/journal.pone.0200824. - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

