Sibjotang Protects against Cardiac Hypertrophy In Vitro and In Vivo
- PMID: 38137908
- PMCID: PMC10744393
- DOI: 10.3390/life13122307
Sibjotang Protects against Cardiac Hypertrophy In Vitro and In Vivo
Abstract
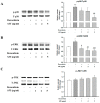
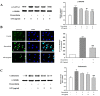
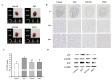
Cardiac hypertrophy is developed by various diseases such as myocardial infarction, valve diseases, hypertension, and aortic stenosis. Sibjotang (, Shizaotang, SJT), a classic formula in Korean traditional medicine, has been shown to modulate the equilibrium of body fluids and blood pressure. This research study sought to explore the impact and underlying process of Sibjotang on cardiotoxicity induced by DOX in H9c2 cells. In vitro, H9c2 cells were induced by DOX (1 μM) in the presence or absence of SJT (1-5 μg/mL) and incubated for 24 h. In vivo, SJT was administrated to isoproterenol (ISO)-induced cardiac hypertrophy mice (n = 8) at 100 mg/kg/day concentrations. Immunofluorescence staining revealed that SJT mitigated the enlargement of H9c2 cells caused by DOX in a dose-dependent way. Using SJT as a pretreatment notably suppressed the rise in cardiac hypertrophic marker levels induced by DOX. SJT inhibited the DOX-induced ERK1/2 and p38 MAPK signaling pathways. In addition, SJT significantly decreased the expression of the hypertrophy-associated transcription factor GATA binding factor 4 (GATA 4) induced by DOX. SJT also decreased hypertrophy-associated calcineurin and NFAT protein levels. Pretreatment with SJT significantly attenuated DOX-induced apoptosis-associated proteins such as Bax, caspase-3, and caspase-9 without affecting cell viability. In addition, the results of the in vivo study indicated that SJT significantly reduced the left ventricle/body weight ratio level. Administration of SJT reduced the expression of hypertrophy markers, such as ANP and BNP. These results suggest that SJT attenuates cardiac hypertrophy and heart failure induced by DOX or ISO through the inhibition of the calcineurin/NFAT/GATA4 pathway. Therefore, SJT may be a potential treatment for the prevention and treatment of cardiac hypertrophy that leads to heart failure.
Keywords: H9c2 cell; apoptosis; heart diseases; heart failure; isoproterenol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Betulinic Acid Protects DOX-Triggered Cardiomyocyte Hypertrophy Response through the GATA-4/Calcineurin/NFAT Pathway.Molecules. 2020 Dec 24;26(1):53. doi: 10.3390/molecules26010053. Molecules. 2020. PMID: 33374365 Free PMC article.
-
TongGuanWan Alleviates Doxorubicin- and Isoproterenol-Induced Cardiac Hypertrophy and Fibrosis by Modulating Apoptotic and Fibrotic Pathways.Int J Mol Sci. 2024 Sep 30;25(19):10573. doi: 10.3390/ijms251910573. Int J Mol Sci. 2024. PMID: 39408900 Free PMC article.
-
Plantago asiatica L. seeds extract protects against cardiomyocyte injury in isoproterenol- induced cardiac hypertrophy by inhibiting excessive autophagy and apoptosis in mice.Phytomedicine. 2021 Oct;91:153681. doi: 10.1016/j.phymed.2021.153681. Epub 2021 Jul 20. Phytomedicine. 2021. PMID: 34371252
-
Zinc inhibits doxorubicin-activated calcineurin signal transduction pathway in H9c2 embryonic rat cardiac cells.Exp Biol Med (Maywood). 2007 May;232(5):682-9. Exp Biol Med (Maywood). 2007. PMID: 17463165
-
Lipopolysaccharide induces cellular hypertrophy through calcineurin/NFAT-3 signaling pathway in H9c2 myocardiac cells.Mol Cell Biochem. 2008 Jun;313(1-2):167-78. doi: 10.1007/s11010-008-9754-0. Epub 2008 Apr 9. Mol Cell Biochem. 2008. PMID: 18398669
Cited by
-
Therapeutic Effects of Natural Products on Human Diseases.Life (Basel). 2024 Sep 14;14(9):1166. doi: 10.3390/life14091166. Life (Basel). 2024. PMID: 39337949 Free PMC article.
-
Research progress on programmed cell death of cardiomyocytes in pressure-overload hypertrophic cardiomyopathy.Apoptosis. 2025 Aug 14. doi: 10.1007/s10495-025-02146-5. Online ahead of print. Apoptosis. 2025. PMID: 40813538 Review.
-
Post-translational acylation of proteins in cardiac hypertrophy.Nat Rev Cardiol. 2025 Apr 14. doi: 10.1038/s41569-025-01150-1. Online ahead of print. Nat Rev Cardiol. 2025. PMID: 40229510 Review.
References
-
- Shimizu I., Minamino T. Myocardial hypertrophy is characterized by the thickening of heart muscles without an obvious cause and found to be involved in several pathological conditions, including hypertension, vascular disease and chronic heart failure. Physiological and pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 2016;97:245–262. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

