Development of a Protocol for Anaerobic Preparation and Banking of Fecal Microbiota Transplantation Material: Evaluation of Bacterial Richness in the Cultivated Fraction
- PMID: 38138045
- PMCID: PMC10745795
- DOI: 10.3390/microorganisms11122901
Development of a Protocol for Anaerobic Preparation and Banking of Fecal Microbiota Transplantation Material: Evaluation of Bacterial Richness in the Cultivated Fraction
Abstract
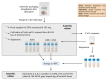
Fecal microbiota transplantation (FMT) has shown highly variable results in indications beyond recurrent Clostridioides difficile infection. Microbiota dysbiosis in many diseases is characterized by the depletion of strictly anaerobic bacteria, which may be crucial for FMT efficacy. We developed a protocol to ensure anaerobic conditions during the entire transplant preparation and banking process, from material collection to administration. The protocol necessitates an anaerobic cabinet, i.e., a non-standard laboratory equipment. We analyzed the population of viable anaerobes by combining cultivation and 16S rRNA gene profiling during the transplant preparation, and after 4, 8, and 12 months of anaerobic or aerobic storage at -80 °C, 78% of fecal species were captured via cultivation. Our findings suggest that strictly anaerobic transplant preparation and storage may preserve species richness better than oxic conditions, but the overall difference was not significant. However, specific anaerobes such as Neglecta and Anaerotruncus were affected by the oxygen exposure. A storage time of up to 12 months did not affect the presence of cultivated taxa. Noteworthy, our analysis focused on the richness of cultivated anaerobes rather than their abundance, which may have been affected. The benefits of the developed anaerobic protocol in FMT for specific indications remain to be demonstrated in clinical trials.
Keywords: Clostridium difficile; anaerobic conditions; dysbiosis; fecal microbiota transplantation (FMT); inflammatory bowel disease; short-chain fatty acid (SCFA).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Xie E., Ensink P., Li J., Gordevičius L.L., Marshall S., George J.A., Pospisilik V.T.E., Aho M.C., Houser P.A.B., Pereira K., et al. Bacterial Butyrate in Parkinson’s Disease Is Linked to Epigenetic Changes and Depressive Symptoms. Mov. Disord. 2022;37:1644–1653. doi: 10.1002/mds.29128. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

