Characterization of Three New Outer Membrane Adhesion Proteins in Fusobacterium necrophorum
- PMID: 38138112
- PMCID: PMC10745669
- DOI: 10.3390/microorganisms11122968
Characterization of Three New Outer Membrane Adhesion Proteins in Fusobacterium necrophorum
Abstract
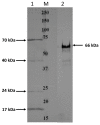
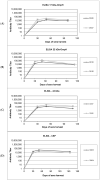
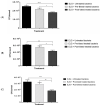
Fusobacterium necrophorum, an anaerobic Gram-negative pathogen, causes necrotic cattle infections, impacting livestock health and the US feedlot industry. Antibiotic administration is the mainstay for treating F. necrophorum infections, although resistance hampers their effectiveness. Vaccination, especially targeting outer membrane proteins (OMPs) due to their antigenic properties and host specificity, offers an alternative to antibiotics. This study identified high-binding-affinity adhesion proteins from F. necrophorum using binding and pull-down assays with bovine adrenal gland endothelial cells (EJG). Four OMP candidates (17.5 kDa/OmpH, 22.7 kDa/OmpA, 66.3 kDa/cell surface protein (CSP), and a previously characterized 43 kDa OMP) were expressed as recombinant proteins and purified. Rabbit polyclonal antibodies to recombinant OMPs were generated, and their ability to inhibit bacterial binding in vitro was assessed. The results show that treatment with individual polyclonal antibodies against 43 kDa significantly inhibited bacterial adhesion, while other antibodies were less potent. However, combinations of two or more antibodies showed a more prominent inhibitory effect on host-cell adhesion. Thus, our findings suggest that the identified OMPs are involved in fusobacterial attachment to host cells and may have the potential to be leveraged in combination for vaccine development. Future in vivo studies are needed to validate their roles and test the feasibility of an OMP-based subunit vaccine against fusobacterial infections.
Keywords: Fusobacterium necrophorum; OmpA; OmpH; cell surface protein; liver abscess; outer membrane proteins (OMPs).
Conflict of interest statement
The authors declare that the research was carried out in the absence of any personal, commercial, or financial relationships that could be construed as potential conflicts of interest.
Figures



Similar articles
-
Adhesion of Fusobacterium necrophorum to bovine endothelial cells is mediated by outer membrane proteins.Vet Microbiol. 2013 Mar 23;162(2-4):813-818. doi: 10.1016/j.vetmic.2012.10.022. Epub 2012 Oct 24. Vet Microbiol. 2013. PMID: 23153522
-
Characterization of Fusobacterium necrophorum subsp. necrophorum outer membrane proteins.Anaerobe. 2018 Apr;50:101-105. doi: 10.1016/j.anaerobe.2018.01.015. Epub 2018 Feb 2. Anaerobe. 2018. PMID: 29408599
-
Screening of BHK-21 cellular proteins that interact with outer membrane protein 43K OMP of Fusobacterium necrophorum.Anaerobe. 2020 Jun;63:102184. doi: 10.1016/j.anaerobe.2020.102184. Epub 2020 Mar 4. Anaerobe. 2020. PMID: 32247918
-
Fusobacterium necrophorum infections: virulence factors, pathogenic mechanism and control measures.Vet Res Commun. 1996;20(2):113-40. doi: 10.1007/BF00385634. Vet Res Commun. 1996. PMID: 8711893 Review.
-
Peritonsillar abscess: clinical aspects of microbiology, risk factors, and the association with parapharyngeal abscess.Dan Med J. 2017 Mar;64(3):B5333. Dan Med J. 2017. PMID: 28260599 Review.
Cited by
-
Pediatric Lemierre's Syndrome: A Comprehensive Literature Review.Pediatr Rep. 2024 Mar 18;16(1):201-213. doi: 10.3390/pediatric16010018. Pediatr Rep. 2024. PMID: 38535514 Free PMC article. Review.
References
-
- Nyack B., Craig I., Padmore C., Bernard N. Necrotic Laryngitis in a Calf. Mod. Vet. Pr. 1981;62:937–938. - PubMed
-
- McCoy E.J., O’Quinn T.G., Schwandt E.F., Reinhardt C.D., Thomson D.U. Liver Abscess Severity at Slaughter Does Not Affect Meat Tenderness and Sensory Attributes in Commercially Finished Beef Cattle Fed Without Tylosin Phosphate. Kans. Agric. Exp. Stn. Res. Rep. 2017;3:33. doi: 10.4148/2378-5977.1367. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

