The Efficacy of Immunotherapy and Clinical Utility of Comprehensive Genomic Profiling in Adenoid Cystic Carcinoma of Head and Neck
- PMID: 38138214
- PMCID: PMC10745089
- DOI: 10.3390/medicina59122111
The Efficacy of Immunotherapy and Clinical Utility of Comprehensive Genomic Profiling in Adenoid Cystic Carcinoma of Head and Neck
Abstract
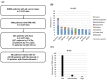
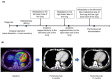
Background and Objectives: Adenoid cystic carcinoma (ACC) of the head and neck is generally slow-growing but has a high potential for local recurrence and metastasis to distant organs. There is currently no standard pharmacological treatment for recurrent/metastatic (R/M) ACC, and there are cases in which immune checkpoint inhibitors (ICIs) are administered for ACC according to head and neck squamous cell carcinoma (HNSCC). However, the efficacy of ICIs for ACC remains unclear, and the predictive biomarkers need to be elucidated. Materials and Methods: The Center for Cancer Genomics and Advanced Therapeutics (C-CAT) database enabled the retrospective but nationwide analysis of 263 cases of ACC of the head and neck. Then, we examined and reported four cases of ACC that received ICIs and comprehensive genomic profiling (CGP) in our institution. Results: The C-CAT database revealed that 59 cases out of 263 received ICIs, and the best response was 8% of objective response rate (ORR) and 53% of disease control rate (DCR) (complete response, CR 3%, partial response, PR 5%, stable disease, SD 44%, progressive disease, PD 19%, not evaluated, NE 29%). The tumor mutational burden (TMB) in ACC was lower overall compared to HNSCC and could not be useful in predicting the efficacy of ICIs. Some cases with MYB structural variants showed the response to ICIs in the C-CAT database. A patient with MYB fusion/rearrangement variants in our institution showed long-term stable disease. Conclusions: ICI therapy is a potential treatment option, and the MYB structural variant might be a candidate for predictive biomarkers for immunotherapy in patients with R/M ACC.
Keywords: C-CAT database; MYB; adenoid cystic carcinoma; comprehensive genomic profiling test; head and neck; immunotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The Immune Landscape of Chinese Head and Neck Adenoid Cystic Carcinoma and Clinical Implication.Front Immunol. 2021 Sep 6;12:618367. doi: 10.3389/fimmu.2021.618367. eCollection 2021. Front Immunol. 2021. PMID: 34552580 Free PMC article.
-
Comprehensive Genomic Profiling Reveals Clinical Associations in Response to Immune Therapy in Head and Neck Cancer.Cancers (Basel). 2022 Jul 18;14(14):3476. doi: 10.3390/cancers14143476. Cancers (Basel). 2022. PMID: 35884537 Free PMC article.
-
Initial analysis of the synergy of programmed cell death-1 (PD-1) inhibitor and concurrent chemoradiotherapy treatment for recurrent/metastatic head and neck squamous cell carcinoma patients.Radiat Oncol. 2023 Jul 4;18(1):109. doi: 10.1186/s13014-023-02310-8. Radiat Oncol. 2023. PMID: 37403098 Free PMC article.
-
Prognosis and management of recurrent and/or metastatic head and neck adenoid cystic carcinoma.Oral Oncol. 2021 Apr;115:105213. doi: 10.1016/j.oraloncology.2021.105213. Epub 2021 Feb 9. Oral Oncol. 2021. PMID: 33578204 Review.
-
The current advances and future directions of PD-1/PD-L1 blockade in head and neck squamous cell carcinoma (HNSCC) in the era of immunotherapy.Int Immunopharmacol. 2023 Jul;120:110329. doi: 10.1016/j.intimp.2023.110329. Epub 2023 May 17. Int Immunopharmacol. 2023. PMID: 37207445 Review.
Cited by
-
Association between homologous recombination deficiency and time to treatment failure to platinum-based chemotherapy for pancreatic cancer by using the C-CAT database.J Gastroenterol. 2025 Feb;60(2):247-256. doi: 10.1007/s00535-024-02173-0. Epub 2024 Nov 21. J Gastroenterol. 2025. PMID: 39570378 Free PMC article.
-
Dynamic Changes in Circulating Tumor DNA During Immunotherapy for Head and Neck Cancer: SHIZUKU-HN Study.Int J Mol Sci. 2024 Dec 30;26(1):235. doi: 10.3390/ijms26010235. Int J Mol Sci. 2024. PMID: 39796090 Free PMC article.
-
Insights into post-marketing clinical validation of companion diagnostics with reference to the FDA, EMA, PMDA, and MFDS.Mol Ther Methods Clin Dev. 2024 Sep 24;32(4):101346. doi: 10.1016/j.omtm.2024.101346. eCollection 2024 Dec 12. Mol Ther Methods Clin Dev. 2024. PMID: 39429725 Free PMC article. Review.
References
-
- Atallah S., Casiraghi O., Fakhry N., Wassef M., Uro-Coste E., Espitalier F., Sudaka A., Kaminsky M.C., Dakpe S., Digue L., et al. A prospective multicentre REFCOR study of 470 cases of head and neck Adenoid cystic carcinoma: Epidemiology and prognostic factors. Eur. J. Cancer. 2020;130:241–249. doi: 10.1016/j.ejca.2020.01.023. - DOI - PubMed
-
- Jeong I.S., Roh J.L., Cho K.J., Choi S.H., Nam S.Y., Kim S.Y. Risk factors for survival and distant metastasis in 125 patients with head and neck adenoid cystic carcinoma undergoing primary surgery. J. Cancer Res. Clin. Oncol. 2020;146:1343–1350. doi: 10.1007/s00432-020-03170-5. - DOI - PMC - PubMed
-
- Lorini L., Ardighieri L., Bozzola A., Romani C., Bignotti E., Buglione M., Guerini A., Lombardi D., Deganello A., Tomasoni M., et al. Prognosis and management of recurrent and/or metastatic head and neck adenoid cystic carcinoma. Oral Oncol. 2021;115:105213. doi: 10.1016/j.oraloncology.2021.105213. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

