Radiation Synthesis of High-Temperature Wide-Bandgap Ceramics
- PMID: 38138362
- PMCID: PMC10745877
- DOI: 10.3390/mi14122193
Radiation Synthesis of High-Temperature Wide-Bandgap Ceramics
Abstract
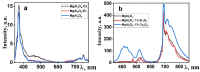
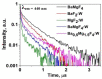
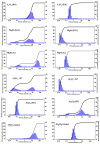
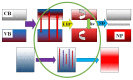
This paper presents the results of ceramic synthesis in the field of a powerful flux of high-energy electrons on powder mixtures. The synthesis is carried out via the direct exposure of the radiation flux to a mixture with high speed (up to 10 g/s) and efficiency without the use of any methods or means for stimulation. These synthesis qualities provide the opportunity to optimize compositions and conditions in a short time while maintaining the purity of the ceramics. The possibility of synthesizing ceramics from powders of metal oxides and fluorides (MgF2, BaF2, WO3, Ga2O3, Al2O3, Y2O3, ZrO2, MgO) and complex compounds from their stoichiometric mixtures (Y3Al3O12, Y3AlxGa(5-x) O12, MgAl2O4, ZnAl2O4, MgWO4, ZnWO4, BaxMg(2-x) F4), including activators, is demonstrated. The ceramics synthesized in the field of high-energy electron flux have a structure and luminescence properties similar to those obtained by other methods, such as thermal methods. The results of studying the processes of energy transfer of the electron beam mixture, quantitative assessments of the distribution of absorbed energy, and the dissipation of this energy are presented. The optimal conditions for beam treatment of the mixture during synthesis are determined. It is shown that the efficiency of radiation synthesis of ceramics depends on the particle dispersion of the initial powders. Powders with particle sizes of 1-10 µm, uniform for the synthesis of ceramics of complex compositions, are optimal. A hypothesis is put forward that ionization processes, resulting in the radiolysis of particles and the exchange of elements in the ion-electron plasma, dominate in the formation of new structural phases during radiation synthesis.
Keywords: ceramics; high-power electron flux; luminescence; radiation synthesis; refractory dielectric materials.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
















References
-
- Liu X., Qian X., Zheng P., Chen X., Feng Y., Shi Y., Zou J., Xie R., Li J. Composition and Structure Design of Three-Layered Composite Phosphors for High Color Rendering Chip-on-Board Light-Emitting Diode Devices. J. Adv. Ceram. 2021;10:729–740. doi: 10.1007/s40145-021-0467-0. - DOI
-
- Li J., Sahi S., Groza M., Pan Y., Burger A., Kenarangui R., Chen W. Optical and Scintillation Properties of Ce3+-Doped LuAG and YAG Transparent Ceramics: A Comparative Study. J. Am. Ceram. Soc. 2017;100:150–156. doi: 10.1111/jace.14461. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

