A Rapid Prototyping Approach for Multi-Material, Reversibly Sealed Microfluidics
- PMID: 38138382
- PMCID: PMC10745384
- DOI: 10.3390/mi14122213
A Rapid Prototyping Approach for Multi-Material, Reversibly Sealed Microfluidics
Abstract
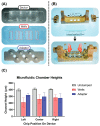
Microfluidic organ-on-chip models recapitulate increasingly complex physiological phenomena to study tissue development and disease mechanisms, where there is a growing interest in retrieving delicate biological structures from these devices for downstream analysis. Standard bonding techniques, however, often utilize irreversible sealing, making sample retrieval unfeasible or necessitating destructive methods for disassembly. To address this, several commercial devices employ reversible sealing techniques, though integrating these techniques into early-stage prototyping workflows is often ignored because of the variation and complexity of microfluidic designs. Here, we demonstrate the concerted use of rapid prototyping techniques, including 3D printing and laser cutting, to produce multi-material microfluidic devices that can be reversibly sealed. This is enhanced via the incorporation of acrylic components directly into polydimethylsiloxane channel layers to enhance stability, sealing, and handling. These acrylic components act as a rigid surface separating the multiple mechanical seals created between the bottom substrate, the microfluidic features in the device, and the fluidic interconnect to external tubing, allowing for greater design flexibility. We demonstrate that these devices can be produced reproducibly outside of a cleanroom environment and that they can withstand ~1 bar pressures that are appropriate for a wide range of biological applications. By presenting an accessible and low-cost method, we hope to enable microfluidic prototyping for a broad range of biomedical research applications.
Keywords: additive manufacturing; lab-on-a-chip; microfluidics; organ-on-chip; rapid prototyping.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Carvalho D.J.T., Moroni L., Giselbrecht S. Clamping strategies for organ-on-a-chip devices. Nat. Rev. Mater. 2023;8:147–164. doi: 10.1038/s41578-022-00523-z. - DOI
-
- Anwar K., Han T., Kim S.M. Reversible sealing techniques for microdevice applications. Sens. Actuators B Chem. 2011;153:301–311. doi: 10.1016/j.snb.2010.11.002. - DOI
-
- Le Berre M., Crozatier C., Casquillas G.V., Chen Y. Reversible assembling of microfluidic devices by aspiration. Microelectron. Eng. 2006;83:1284–1287. doi: 10.1016/j.mee.2006.01.257. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

