NMR and Docking Calculations Reveal Novel Atomistic Selectivity of a Synthetic High-Affinity Free Fatty Acid vs. Free Fatty Acids in Sudlow's Drug Binding Sites in Human Serum Albumin
- PMID: 38138481
- PMCID: PMC10745614
- DOI: 10.3390/molecules28247991
NMR and Docking Calculations Reveal Novel Atomistic Selectivity of a Synthetic High-Affinity Free Fatty Acid vs. Free Fatty Acids in Sudlow's Drug Binding Sites in Human Serum Albumin
Abstract
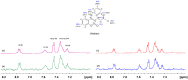
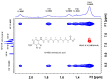
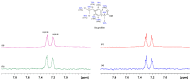
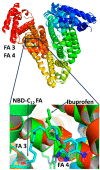
Saturation transfer difference (STD), inter-ligand NOEs (INPHARMA NMR), and docking calculations are reported for investigating specific binding sites of the high-affinity synthetic 7-nitrobenz-2-oxa-1,3-diazoyl-4-C12 fatty acid (NBD-C12 FA) with non-labeled human serum albumin (HSA) and in competition with the drugs warfarin and ibuprofen. A limited number of negative interligand NOEs between NBD-C12 FA and warfarin were interpreted in terms of a short-range allosteric competitive binding in the wide Sudlow's binding site II (FA7) of NBD-C12 FA with Ser-202, Lys-199, and Trp-214 and warfarin with Arg-218 and Arg-222. In contrast, the significant number of interligand NOEs between NBD-C12 FA and ibuprofen were interpreted in terms of a competitive binding mode in Sudlow's binding site I (FA3 and FA4) with Ser-342, Arg-348, Arg-485, Arg-410, and Tyr-411. NBD-C12 FA has the unique structural properties, compared to short-, medium-, and long-chain saturated and unsaturated natural free fatty acids, of interacting with well-defined structures with amino acids of both the internal and external polar anchor sites in Sudlow's binding site I and with amino acids in both FA3 and FA4 in Sudlow's binding site II. The NBD-C12 FA, therefore, interacts with novel structural characteristics in the drug binding sites I and II and can be regarded as a prototype molecule for drug development.
Keywords: HSA; INPHARMA NMR; NBD-C12 FA; STD NMR; docking calculations.
Conflict of interest statement
S.P. is an employee and shareholder of Sanofi. The other authors declare no conflict of interest.
Figures



References
-
- Hodgson J. All about Albumin: Biochemistry, Genetics and Medical Applications. Academic Press; San Diego, CA, USA: 2001.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

