Shock-Induced Degradation of Guanosine and Uridine Promoted by Nickel and Carbonate: Potential Applications
- PMID: 38138495
- PMCID: PMC10745911
- DOI: 10.3390/molecules28248006
Shock-Induced Degradation of Guanosine and Uridine Promoted by Nickel and Carbonate: Potential Applications
Abstract
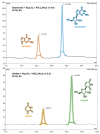
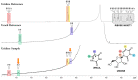
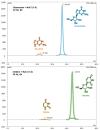
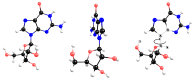
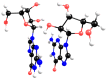
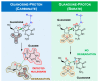
Experimental studies of the degradation of two ribonucleosides (guanosine and uridine) were carried out by making use of mechanochemistry. Mechanochemical experiments reveal the decomposition of guanosine and uridine, promoted by nickel(II) and carbonate ions, into guanine and uracil, respectively. These nucleobases were identified by HPLC and 1H NMR spectroscopy (this applied only to uracil). Additionally, density-functional theory (DFT) methodologies were used to probe the energetic viability of several degradation pathways, including in the presence of the abovementioned ions. Three mechanisms were analysed via ribose ring-opening: dry, single-molecule water-assisted, and metal-assisted, wherein the last two mechanisms confirmed the mechanochemical degradation of both ribonucleosides into respective nucleobase moieties. These results can contribute to an astrobiological interpretation of the extraterrestrial sample's contents.
Keywords: DFT; asteroids; degradation reactions; mechanochemistry; meteorites.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Uridine and cytidine transport in Escherichia coli B and transport-deficient mutants.J Biol Chem. 1977 Apr 25;252(8):2492-7. J Biol Chem. 1977. PMID: 323246
-
Pyridyl groups for protection of the imide functions of uridine and guanosine. Exploration of their displacement reactions for site-specific modifications of uracil and guanine bases.Acta Chem Scand B. 1986 Nov;40(10):806-16. doi: 10.3891/acta.chem.scand.40b-0806. Acta Chem Scand B. 1986. PMID: 3564801
-
Extraterrestrial ribose and other sugars in primitive meteorites.Proc Natl Acad Sci U S A. 2019 Dec 3;116(49):24440-24445. doi: 10.1073/pnas.1907169116. Epub 2019 Nov 18. Proc Natl Acad Sci U S A. 2019. PMID: 31740594 Free PMC article.
-
Abiotic synthesis of purine and pyrimidine ribonucleosides in aqueous microdroplets.Proc Natl Acad Sci U S A. 2018 Jan 2;115(1):36-40. doi: 10.1073/pnas.1718559115. Epub 2017 Dec 18. Proc Natl Acad Sci U S A. 2018. PMID: 29255025 Free PMC article.
-
Wandering through quantum-mechanochemistry: from concepts to reactivity and switches.Phys Chem Chem Phys. 2023 Dec 21;26(1):21-35. doi: 10.1039/d3cp04907h. Phys Chem Chem Phys. 2023. PMID: 38086672 Review.
References
-
- Pagola S. Outstanding Advantages, Current Drawbacks, and Significant Recent Developments in Mechanochemistry: A Perspective View. Crystals. 2023;13:124. doi: 10.3390/cryst13010124. - DOI
-
- Kaupp G.J.C. Mechanochemistry: The varied applications of mechanical bond-breaking. CrystEngComm. 2009;11:388–403. doi: 10.1039/B810822F. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

