Assessing the Novel Mixed Tutton Salts K2Mn0.03Ni0.97(SO4)2(H2O)6 and K2Mn0.18Cu0.82(SO4)2(H2O)6 for Thermochemical Heat Storage Applications: An Experimental-Theoretical Study
- PMID: 38138548
- PMCID: PMC10745892
- DOI: 10.3390/molecules28248058
Assessing the Novel Mixed Tutton Salts K2Mn0.03Ni0.97(SO4)2(H2O)6 and K2Mn0.18Cu0.82(SO4)2(H2O)6 for Thermochemical Heat Storage Applications: An Experimental-Theoretical Study
Abstract
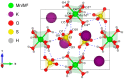
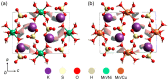
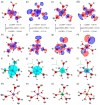
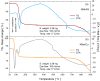
In this paper, novel mixed Tutton salts with the chemical formulas K2Mn0.03Ni0.97(SO4)2(H2O)6 and K2Mn0.18Cu0.82(SO4)2(H2O)6 were synthesized and studied as compounds for thermochemical heat storage potential. The crystallographic structures of single crystals were determined by X-ray diffraction. Additionally, a comprehensive computational study, based on density functional theory (DFT) calculations and Hirshfeld surface analysis, was performed to calculate structural, electronic, and thermodynamic properties of the coordination complexes [MII(H2O)6]2+ (MII = Mn, Ni, and Cu), as well as to investigate intermolecular interactions and voids in the framework. The axial compressions relative to octahedral coordination geometry observed in the crystal structures were correlated and elucidated using DFT investigations regarding Jahn-Teller effects arising from complexes with different spin multiplicities. The spatial distributions of the frontier molecular orbital and spin densities, as well as energy gaps, provided further insights into the stability of these complexes. Thermogravimetry, differential thermal analysis, and differential scanning calorimetry techniques were also applied to identify the thermal stability and physicochemical properties of the mixed crystals. Values of dehydration enthalpy and storage energy density per volume were also estimated. The two mixed sulfate hydrates reported here have low dehydration temperatures and high energy densities. Both have promising thermal properties for residential heat storage systems, superior to the Tutton salts previously reported.
Keywords: DFT calculations; Hirshfeld fingerprint plots; mixed Tutton salts; single-crystal growth; thermochemical compounds.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Barpanda P., Oyama G., Ling C.D., Yamada A. Kröhnkite-Type Na2Fe(SO4)2·2H2O as a Novel 3.25 V Insertion Compound for Na-Ion Batteries. Chem. Mater. 2014;26:1297–1299. doi: 10.1021/cm4033226. - DOI
-
- Ousaleh H.A., Sair S., Zaki A., Faik A., Igartua J.M., El Bouari A. Double hydrates salt as sustainable thermochemical energy storage materials: Evaluation of dehydration behavior and structural phase transition reversibility. Sol. Energy. 2020;201:846–856. doi: 10.1016/j.solener.2020.03.067. - DOI
-
- Kooijman W., Kok D., Blijlevens M., Meekes H., Vlieg E. Screening double salt sulfate hydrates for application in thermochemical heat storage. J. Energy Storage. 2022;55:105459. doi: 10.1016/j.est.2022.105459. - DOI
-
- Wang X., Zhuang X., Su G., He Y. A new ultraviolet filter: Rb2Ni(SO4)2·6H2O (RNSH) single crystal. Opt. Mater. 2008;31:233–236. doi: 10.1016/j.optmat.2008.03.020. - DOI
-
- Souamti A., Martín I., Zayani L., Lozano-Gorrín A., Chehimi D.B.H. Luminescence properties of Pr3+ ion doped Mg-picromerite Tutton salt. J. Lumin. 2017;188:148–153. doi: 10.1016/j.jlumin.2017.04.022. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

