Oxidation of Mesalamine under Phenoloxidase- or Peroxidase-like Enzyme Catalysis
- PMID: 38138595
- PMCID: PMC10871084
- DOI: 10.3390/molecules28248105
Oxidation of Mesalamine under Phenoloxidase- or Peroxidase-like Enzyme Catalysis
Abstract
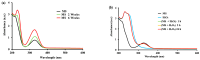
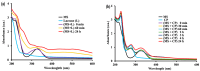
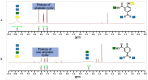
Mesalamine, also called 5-ASA (5-aminosalicylic acid), is a largely used anti-inflammatory agent and is a main choice to treat Ulcerative Colitis. This report is aimed to investigate enzymatic processes involved in the oxidation of mesalamine to better understand some of its side-effects. Oxidation with oxygen (catalyzed by ceruloplasmin) or with hydrogen peroxide (catalyzed by peroxidase or hemoglobin) showed that these oxidases, despite their different mechanisms of oxidation, could recognize mesalamine as a substrate and trigger its oxidation to a corresponding quinone-imine. These enzymes were chosen because they may recognize hydroquinone (a p-diphenol) as substrate and oxidize it to p-benzoquinone and that mesalamine, as a p-aminophenol, presents some similarities with hydroquinone. The UV-Vis kinetics, FTIR and 1H NMR supported the hypothesis of oxidizing mesalamine. Furthermore, mass spectrometry suggested the quinone-imine as reaction product. Without enzymes, the oxidation process was very slow (days and weeks), but it was markedly accelerated with the oxidases, particularly with peroxidase. Cyclic voltammetry supported the hypothesis of the oxidative process and allowed a ranking of susceptibility to oxidizing mesalamine in comparison with other oxidizable drug molecules with related structures. The susceptibility to oxidation was higher for mesalamine, in comparison with Tylenol (acetaminophen) and with aspirin (salicylic acid).
Keywords: 5-ASA (5-aminosalicylic acid); acetaminophen; ceruloplasmin; hemoglobin; hydrogen peroxide; hydroquinone; inflammatory bowel diseases; laccase; mesalamine; peroxidase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Nikias G., Eisner T., Katz S., Levin L., Eskries D., Urmacher C., McKinley M. Crohn’s disease and colorectal carcinoma: Rectal cancer complicating longstanding active perianal disease. Am. J. Gastroenterol. 1995;90:216–219. - PubMed
-
- Abdu-Allah H.H., El-Shorbagi A., Abdel-Moty S.G., El-Awady R., Abdel-Alim A. 5-Aminosalyclic acid (5-ASA): A unique anti-inflammatory salicylate. Med. Chem. 2016;6:306–315. doi: 10.4172/2161-0444.1000361. - DOI
-
- Mesalazine European Pharmacopoeia (EP) Reference Standard. Y0000297. [(accessed on 19 October 2023)]. Available online: http://www.uspbpep.com/ep50/Mesalazine.pdf.
-
- Oostlander A.E., Everts V., Schoenmaker T., Bravenboer N., van Vliet S.J., van Bodegraven A.A., Lips P., de Vries T.J. T cell-mediated increased osteoclast formation from peripheral blood as a mechanism for crohn’s disease-associated bone loss. J. Cell. Biochem. 2012;113:260–268. doi: 10.1002/jcb.23352. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

