Should We Abandon Intraperitoneal Chemotherapy in the Treatment of Advanced Ovarian Cancer? A Meta-Analysis
- PMID: 38138863
- PMCID: PMC10745120
- DOI: 10.3390/jpm13121636
Should We Abandon Intraperitoneal Chemotherapy in the Treatment of Advanced Ovarian Cancer? A Meta-Analysis
Abstract
Background: Ovarian cancer is the gynaecological malignancy with the highest mortality and diagnosis often occurs in its advanced stages. Standard treatment in these cases is based on complete cytoreductive surgery with adjuvant intravenous chemotherapy. Other types of treatment are being evaluated to improve the prognosis of these patients, including intraperitoneal chemotherapy and antiangiogenic therapy. These may improve survival or time to relapse in addition to intravenous chemotherapy.
Objective: The aim of this meta-analysis is to determine whether treatment with intravenous chemotherapy remains the gold standard, or whether the addition of intraperitoneal chemotherapy has a benefit in overall survival (OS) and disease-free interval (DFS).
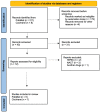
Materials and methods: A literature search was carried out in Pubmed and Cochrane, selecting clinical studies and systematic reviews published in the last 10 years. Statistical analysis was performed using the hazard ratio measure in the RevMan tool.
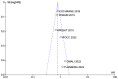
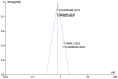
Results: Intraperitoneal chemotherapy shows a benefit in OS and DFS compared with standard intravenous chemotherapy. The significant differences in OS (HR: 0.81 CI 95% 0.74-0.88) and in DFS (HR: 0.81 CI 95% 0.75-0.87) are statistically significant (p < 0.00001). There were no clinical differences in toxicity and side-effects.
Conclusion: Intraperitoneal chemotherapy is an option that improves OS and DFS without significant toxicity regarding the use of intravenous chemotherapy alone. However, prospective studies are needed to determine the optimal dose and treatment regimen that will maintain the benefits while minimising side effects and toxicity and the profile of patients who will benefit most from this treatment.
Keywords: advanced ovarian cancer; disease-free survival; intraperitoneal chemotherapy; overall survival; primary cytorreductive surgery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bookman M.A., Brady M.F., McGuire W.P., Harper P.G., Alberts D.S., Friedlander M., Colombo N., Fowler J.M., Argenta P.A., Geest K.D., et al. Evaluation of New Platinum-Based Treatment Regimens in Advanced-Stage Ovarian Cancer: A Phase III Trial of the Gynecologic Cancer InterGroup. J. Clin. Oncol. 2009;27:1419. doi: 10.1200/JCO.2008.19.1684. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

