Reduced Lipopolysaccharide-Binding Protein (LBP) Levels Are Associated with Non-Alcoholic Fatty Liver Disease (NAFLD) and Adipose Inflammation in Human Obesity
- PMID: 38139003
- PMCID: PMC10742626
- DOI: 10.3390/ijms242417174
Reduced Lipopolysaccharide-Binding Protein (LBP) Levels Are Associated with Non-Alcoholic Fatty Liver Disease (NAFLD) and Adipose Inflammation in Human Obesity
Abstract
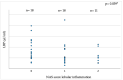
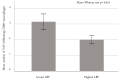
Lipopolysaccharide (LPS) and its binding protein LBP have emerged as potential contributors to the progression from overweight/obesity to overt metabolic diseases and NAFLD. While LPS is known to activate hepatocyte inflammation, thus contributing toward NAFLD development, the role of LBP is more intricate, and recent data have shown that experimental reduction in hepatic LBP promotes NAFLD progression. In this cross-sectional investigation, we evaluated circulating LBP in relation to obesity, NAFLD, visceral adipose tissue (VAT) inflammation, and type 2 diabetes (T2D). We recruited 186 individuals (M/F: 81/105; age: 47 ± 10.4 years; BMI: 35.5 ± 8.6 kg/m2); a subgroup (n = 81) underwent bariatric surgery with intra-operative VAT and liver biopsies. LBP levels were higher in obese individuals than non-obese individuals but were inversely correlated with the parameters of glucose metabolism. Reduced LBP predicted T2D independent of age, sex, and BMI (p < 0.001). LBP levels decreased across more severe stages of hepatosteatosis and lobular inflammation, and were inversely associated with VAT inflammation signatures. In conclusion, LBP levels are increased in obese individuals and are associated with a more favorable metabolic profile and lower NAFLD/NASH prevalence. A possible explanation for these findings is that hepatic LBP production may be triggered by chronic caloric excess and facilitate LPS degradation in the liver, thus protecting these individuals from the metabolic consequences of obesity.
Keywords: MAFLD; MASLD; NASH; insulin resistance; liver fibrosis; low-grade inflammation; metabolic syndrome; type 2 diabetes; visceral obesity.
Conflict of interest statement
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Eslam M., Newsome P.N., Sarin S.K., Anstee Q.M., Targher G., Romero-Gomez M., Zelber-Sagi S., Wong V.W.-S., Dufour J.-F., Schattenberg J.M., et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

