The Role of Neutrophils in ANCA-Associated Vasculitis: The Pathogenic Role and Diagnostic Utility of Autoantibodies
- PMID: 38139045
- PMCID: PMC10743134
- DOI: 10.3390/ijms242417217
The Role of Neutrophils in ANCA-Associated Vasculitis: The Pathogenic Role and Diagnostic Utility of Autoantibodies
Abstract
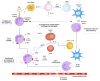
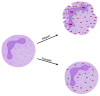
Recent years have brought progress in understanding the role of the neutrophil, dispelling the dogma of homogeneous cells mainly involved in the prime defence against pathogens, shedding light on their pathogenic role in inflammatory diseases and on the importance of antineutrophil-cytoplasmic antibodies' pathogenic role in ANCA-associated vasculitides vasculitis (AAV). Myeloperoxidase (MPO) and proteinase 3 (PR3) expressed in neutrophil granulocytes are the most common targets for ANCAs and contribute to the formation of MPO-ANCAs and PR3-ANCAs which, released to the bloodstream, become an excellent diagnostic tool for AAV. In this study, we focus on increasing the clinical and experimental evidence that supports the pathogenic role of ANCAs in AAV. Additionally, we discuss the diagnostic utility of ANCAs for disease activity and prognosis in AAV. Understanding the central role of ANCAs in AAV is crucial for advancing our knowledge of these complex disorders and developing targeted therapeutic strategies in the era of personalized medicine.
Keywords: ANCA-associated vasculitis (AAV); antineutrophil-cytoplasmic antibodies (ANCAs); neutrophil; proteinase 3-/ myeloperoxidase-ANCA; vasculitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

