Sialyltransferase Mutations Alter the Expression of Calcium-Binding Interneurons in Mice Neocortex, Hippocampus and Striatum
- PMID: 38139047
- PMCID: PMC10743413
- DOI: 10.3390/ijms242417218
Sialyltransferase Mutations Alter the Expression of Calcium-Binding Interneurons in Mice Neocortex, Hippocampus and Striatum
Abstract
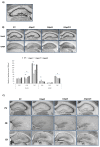
Gangliosides are major glycans on vertebrate nerve cells, and their metabolic disruption results in congenital disorders with marked cognitive and motor deficits. The sialyltransferase gene St3gal2 is responsible for terminal sialylation of two prominent brain gangliosides in mammals, GD1a and GT1b. In this study, we analyzed the expression of calcium-binding interneurons in primary sensory (somatic, visual, and auditory) and motor areas of the neocortex, hippocampus, and striatum of St3gal2-null mice as well as St3gal3-null and St3gal2/3-double null. Immunohistochemistry with highly specific primary antibodies for GABA, parvalbumin, calretinin, and calbindin were used for interneuron detection. St3gal2-null mice had decreased expression of all three analyzed types of calcium-binding interneurons in all analyzed regions of the neocortex. These results implicate gangliosides GD1a and GT1b in the process of interneuron migration and maturation.
Keywords: St3gal2; St3gal3; calcium-binding interneurons; cortex; hippocampus; striatum.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Neurod6 Expression Defines New Retinal Amacrine Cell Subtypes and Regulates Their Fate|Nature Neuroscience. [(accessed on 22 June 2022)]. Available online: https://www.nature.com/articles/nn.2859. - PMC - PubMed
-
- DeFelipe J., López-Cruz P.L., Benavides-Piccione R., Bielza C., Larrañaga P., Anderson S., Burkhalter A., Cauli B., Fairén A., Feldmeyer D., et al. New Insights into the Classification and Nomenclature of Cortical GABAergic Interneurons. Nat. Rev. Neurosci. 2013;14:202–216. doi: 10.1038/nrn3444. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

