Antarctic Krill Oil from Euphausia superba Ameliorates Carrageenan-Induced Thrombosis in a Mouse Model
- PMID: 38139268
- PMCID: PMC10743491
- DOI: 10.3390/ijms242417440
Antarctic Krill Oil from Euphausia superba Ameliorates Carrageenan-Induced Thrombosis in a Mouse Model
Abstract
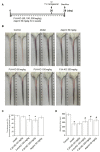
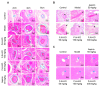
FJH-KO obtained from Antarctic krill, especially Euphausia superba, has been reported to contain high amounts of omega-3 polyunsaturated fatty acids (n-3 PUFA) and to exhibit anticancer and anti-inflammatory properties. However, its antithrombotic effects have not yet been reported. This study aimed to investigate the antithrombotic effects of FJH-KO in carrageenan-induced thrombosis mouse models and human endothelial cells. Thrombosis was induced by carrageenan injection, whereas the mice received FJH-KO pretreatment. FJH-KO attenuated carrageenan-induced thrombus formation in mouse tissue vessels and prolonged tail bleeding. The inhibitory effect of FJH-KO was associated with decreased plasma levels of thromboxane B2, P-selectin, endothelin-1, β-thromboglobulin, platelet factor 4, serotonin, TNF-α, IL-1β, and IL-6. Meanwhile, FJH-KO induced plasma levels of prostacyclin I2 and plasminogen. In vitro, FJH-KO decreased the adhesion of THP-1 monocytes to human endothelial cells stimulated by TNF-α via eNOS activation and NO production. Furthermore, FJH-KO inhibited the expression of TNF-α-induced adhesion molecules such as ICAM-1 and VCAM-1 by suppressing the NF-κB signaling pathway. Taken together, our study demonstrates that FJH-KO protects against carrageenan-induced thrombosis by regulating endothelial cell activation and has potential as an antithrombotic agent.
Keywords: carrageenan; endothelial dysfunction; inflammation; krill oil; thrombosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., Barengo N.C., Beaton A.Z., Benjamin E.J., Benziger C.P., et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

