A Stable Micellar Formulation of RAD001 for Intracerebroventricular Delivery and the Treatment of Alzheimer's Disease and Other Neurological Disorders
- PMID: 38139306
- PMCID: PMC10744130
- DOI: 10.3390/ijms242417478
A Stable Micellar Formulation of RAD001 for Intracerebroventricular Delivery and the Treatment of Alzheimer's Disease and Other Neurological Disorders
Abstract
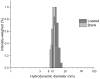
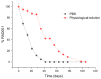
A large body of evidence, replicated in many mouse models of Alzheimer's disease (AD), supports the therapeutic efficacy of the oral mammalian target of rapamycin inhibitors (mTOR-Is). Our preliminary data show that intracerebroventricular (ICV) administration of everolimus (RAD001) soon after clinical onset greatly diminished cognitive impairment and the intracellular beta amyloid and neurofibrillary tangle load. However, RAD001 shows >90% degradation after 7 days in solution at body temperature, thus hampering the development of proper therapeutic regimens for patients. To overcome such a drawback, we developed a stable, liquid formulation of mTOR-Is by loading RAD001 into distearoylphosphatidylethanolamine-polyethylene glycol 2000 (DSPE-PEG2000) micelles using the thin layer evaporation method. The formulation showed efficient encapsulation of RAD001 and a homogeneous colloidal size and stabilised RAD001, with over 95% of activity preserved after 14 days at 37 °C with a total decay only occurring after 98 days. RAD001-loaded DSPE-PEG2000 micelles were unchanged when stored at 4 and 25 °C over the time period investigated. The obtained formulation may represent a suitable platform for expedited clinical translation and effective therapeutic regimens in AD and other neurological diseases.
Keywords: Alzheimer’s disease; DSPE-PEG2000 micelles; drug stabilisation; intracerebroventricular; mTOR; mTOR-I; micellar liquid formulation; neurodegeneration; neurological disorders; rapalog.
Conflict of interest statement
D.D., S.G. and A.M. are co-authors of the patent WO2021205297 (A1). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

