Monofluoromethylation of N-Heterocyclic Compounds
- PMID: 38139426
- PMCID: PMC10744182
- DOI: 10.3390/ijms242417593
Monofluoromethylation of N-Heterocyclic Compounds
Abstract
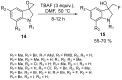
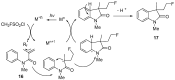
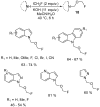
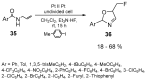
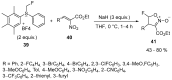
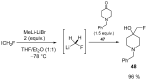
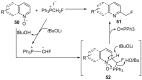
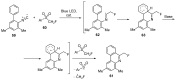
The review focuses on recent advances in the methodologies for the formation or introduction of the CH2F moiety in N-heterocyclic substrates over the past 5 years. The monofluoromethyl group is one of the most versatile fluorinated groups used to modify the properties of molecules in synthetic medical chemistry. The review summarizes two strategies for the monofluoromethylation of N-containing heterocycles: direct monofluoromethylation with simple XCH2F sources (for example, ICH2F) and the assembly of N-heterocyclic structures from CH2F-containing substrates. The review describes the monofluoromethylation of pharmaceutically important three-, five- and six-membered N-heterocycles: pyrrolidines, pyrroles, indoles, imidazoles, triazoles, benzothiazoles, carbazoles, indazoles, pyrazoles, oxazoles, piperidines, morpholines, pyridines, quinolines and pyridazines. Assembling of 6-fluoromethylphenanthridine, 5-fluoromethyl-2-oxazolines, C5-monofluorinated isoxazoline N-oxides, and α-fluoromethyl-α-trifluoromethylaziridines is also shown. Fluoriodo-, fluorchloro- and fluorbromomethane, FCH2SO2Cl, monofluoromethyl(aryl)sulfoniummethylides, monofluoromethyl sulfides, (fluoromethyl)triphenylphosphonium iodide and 2-fluoroacetic acid are the main fluoromethylating reagents in recent works. The replacement of atoms and entire functional groups with a fluorine atom(s) leads to a change and often improvement in activity, chemical or biostability, and pharmacokinetic properties. The monofluoromethyl group is a bioisoster of -CH3, -CH2OH, -CH2NH2, -CH2CH3, -CH2NO2 and -CH2SH moieties. Bioisosteric replacement with the CH2F group is both an interesting task for organic synthesis and a pathway to modify drugs, agrochemicals and useful intermediates.
Keywords: fluorination; fluorine; fluoromethylation; imidazoles; indoles; nitrogen heterocyles; pyridazines; pyridines; pyrrolidines; quinalines.
Conflict of interest statement
The author declares no conflict of interest.
Figures


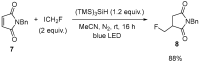
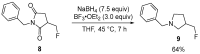


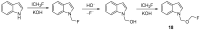





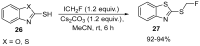

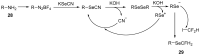


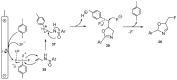
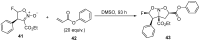
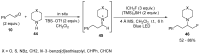

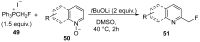
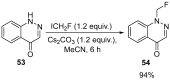

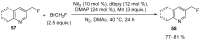
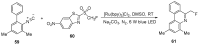



Similar articles
-
Selective difluoromethylation and monofluoromethylation reactions.Chem Commun (Camb). 2009 Dec 28;(48):7465-78. doi: 10.1039/b916463d. Epub 2009 Oct 30. Chem Commun (Camb). 2009. PMID: 20024251
-
Reagents for Selective Fluoromethylation: A Challenge in Organofluorine Chemistry.Angew Chem Int Ed Engl. 2020 Jul 20;59(30):12268-12281. doi: 10.1002/anie.201913175. Epub 2020 Jun 4. Angew Chem Int Ed Engl. 2020. PMID: 32022357 Free PMC article. Review.
-
Recent developments in the synthesis of nitrogen-containing heterocycles from β-aminovinyl esters/ketones as CC-N donors.Org Biomol Chem. 2022 Jan 5;20(2):282-295. doi: 10.1039/d1ob01998h. Org Biomol Chem. 2022. PMID: 34877952 Review.
-
Fluoromethoxymethylation of Nitrogen Heterocyclic Compounds with Fluoromethyl Iodide.J Org Chem. 2020 Mar 20;85(6):3993-4001. doi: 10.1021/acs.joc.9b03193. Epub 2020 Jan 14. J Org Chem. 2020. PMID: 31913626
-
Recent advances in catalytic enantioselective construction of monofluoromethyl-substituted stereocenters.Chem Commun (Camb). 2024 Oct 22;60(85):12302-12314. doi: 10.1039/d4cc03788j. Chem Commun (Camb). 2024. PMID: 39240236 Review.
Cited by
-
Radical Fluoromethylation Enabled by Cobalamin-Dependent Radical SAM Enzymes.ACS Bio Med Chem Au. 2025 May 6;5(3):464-474. doi: 10.1021/acsbiomedchemau.5c00062. eCollection 2025 Jun 18. ACS Bio Med Chem Au. 2025. PMID: 40556783 Free PMC article.
-
Photo-induced decarboxylative radical cascade cyclization of unactivated alkenes: access to CF- and CF2-substituted ring-fused imidazoles.RSC Adv. 2025 Apr 22;15(16):12739-12745. doi: 10.1039/d5ra02023a. eCollection 2025 Apr 16. RSC Adv. 2025. PMID: 40264862 Free PMC article.
-
Linking Fluorine with Bio-Derived Furfural: Aiming Towards More Sustainable Fluorinated Polymers and Drugs.Molecules. 2025 May 24;30(11):2305. doi: 10.3390/molecules30112305. Molecules. 2025. PMID: 40509193 Free PMC article. Review.
References
-
- Barreca M., Spanò V., Rocca R., Bivacqua R., Gualtieri G., Raimondi M.V., Gaudio E., Bortolozzi R., Manfreda L., Bai R., et al. Identification of pyrrolo[3′,4′:3,4]cyclohepta[1,2-d][1,2]oxazoles as promising new candidates for the treatment of lymphomas. Eur. J. Med. Chem. 2023;254:115372. doi: 10.1016/j.ejmech.2023.115372. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

