A Compact Monitor for Ethylene and Other Plant-Produced Volatile Organic Compounds for NASA Space Missions
- PMID: 38139559
- PMCID: PMC10747848
- DOI: 10.3390/s23249713
A Compact Monitor for Ethylene and Other Plant-Produced Volatile Organic Compounds for NASA Space Missions
Abstract
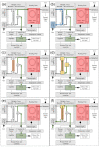
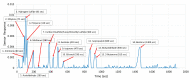
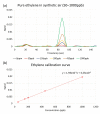
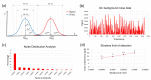
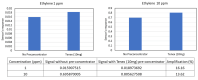
In this work, we discuss the development of a compact analytical instrument for monitoring ethylene in compact greenhouses utilized by NASA to grow fresh vegetables in space. Traditionally, ethylene measurements are conducted by GC-MS systems. However, in space, they are not applicable due to their bulky size, heavy weight, special carrier gas requirement and high maintenance. Our group developed a compact and robust battery-powered ethylene monitor based on the principles of analytical gas chromatography. The device utilizes purified ambient air as a carrier gas and a metal oxide sensor as a GC detector. Implementation of a CarboWax 20 M packed column from Restek together with a Tenax TA pre-concentrator allowed us to achieve a 20 ppb limit of detection for ethylene. Full automation of measurements and reporting of concentrations was accomplished via the implementation of a Raspberry Pi 4 computer and a 7″ 720P LED capacitive touchscreen utilized for data output. Based on a feasibility study, a fully automated, industrial-grade ethylene monitoring and removal system for greenhouses was developed.
Keywords: chemical sensors; ethylene; gas chromatography; volatile organic compounds.
Conflict of interest statement
Authors Alexander Larin and Andrey Livchak were employed by the company Halton Group, and authors Elena Viugina, Jay T. Cremer, Jr. and Charles K. Gary were employed by the company Adelphi Technology LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









References
-
- Salisbury F.B., Campbell W.F., Carman J.G., Bingham G.E., Bubenheim D.L., Yendler B., Sytchev V., Levinskikh M.A., Ivanova I., Chernova L., et al. Plant growth during the greenhouse II experiment on the Mir orbital station. Adv. Space Res. 2003;31:221–227. doi: 10.1016/S0273-1177(02)00744-5. - DOI - PubMed
-
- Eckart P. Life Support and Biospherics. Herbert Utz Publishers; Munchen, Germany: 1994.
-
- Perino M.A., Lobascio C., Pastor S., Maffei M. Greenhouse: A strategic element to support humans in space; Proceedings of the Seventh ISU Annual International Symposium; Strasbourg, France. 4–7 June 2002.
-
- Wang Z., Fingas M., Yang C., Christensen J.H. Crude Oil and Refined Product Fingerprinting: Principles. In: Morrison R.D., Murphy B.L., editors. Environmental Forensics. Academic Press; Burlington, MA, USA: 2005. pp. 339–407.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

