Evaluation of a Peptide Hydrogel as a Chondro-Instructive Three-Dimensional Microenvironment
- PMID: 38139882
- PMCID: PMC10747086
- DOI: 10.3390/polym15244630
Evaluation of a Peptide Hydrogel as a Chondro-Instructive Three-Dimensional Microenvironment
Abstract
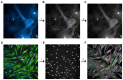
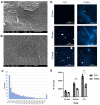
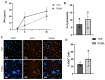
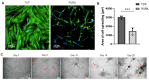
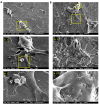
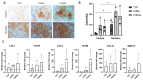
Articular cartilage injuries are inherently irreversible, even with the advancement in current therapeutic options. Alternative approaches, such as the use of mesenchymal stem/stromal cells (MSCs) and tissue engineering techniques, have gained prominence. MSCs represent an ideal source of cells due to their low immunogenicity, paracrine activity, and ability to differentiate. Among biomaterials, self-assembling peptide hydrogels (SAPH) are interesting given their characteristics such as good biocompatibility and tunable properties. Herein we associate human adipose-derived stem cells (hASCs) with a commercial SAPH, Puramatrix™, to evaluate how this three-dimensional microenvironment affects cell behavior and its ability to undergo chondrogenic differentiation. We demonstrate that the Puramatrix™ hydrogel comprises a highly porous matrix permissible for hASC adhesion and in vitro expansion. The morphology and cell growth dynamics of hASCs were affected when cultured on the hydrogel but had minimal alteration in their immunophenotype. Interestingly, hASCs spontaneously formed cell aggregates throughout culturing. Analysis of glycosaminoglycan production and gene expression revealed a noteworthy and donor-dependent trend suggesting that Puramatrix™ hydrogel may have a natural capacity to support the chondrogenic differentiation of hASCs. Altogether, the results provide a more comprehensive understanding of the potential applications and limitations of the Puramatrix™ hydrogel in developing functional cartilage tissue constructs.
Keywords: Puramatrix; adipose-derived stem cells; chondrogenic differentiation; peptide hydrogels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

