Pharmacokinetic Study of Islatravir and Etonogestrel Implants in Macaques
- PMID: 38140017
- PMCID: PMC10747562
- DOI: 10.3390/pharmaceutics15122676
Pharmacokinetic Study of Islatravir and Etonogestrel Implants in Macaques
Abstract
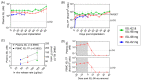
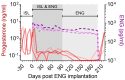
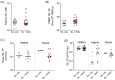
The prevention of HIV and unintended pregnancies is a public health priority. Multi-purpose prevention technologies capable of long-acting HIV and pregnancy prevention are desirable for women. Here, we utilized a preclinical macaque model to evaluate the pharmacokinetics of biodegradable ε-polycaprolactone implants delivering the antiretroviral islatravir (ISL) and the contraceptive etonogestrel (ENG). Three implants were tested: ISL-62 mg, ISL-98 mg, and ENG-33 mg. Animals received one or two ISL-eluting implants, with doses of 42, 66, or 108 µg of ISL/day with or without an additional ENG-33 mg implant (31 µg/day). Drug release increased linearly with dose with median [range] plasma ISL levels of 1.3 [1.0-2.5], 1.9 [1.2-6.3] and 2.8 [2.3-11.6], respectively. The ISL-62 and 98 mg implants demonstrated stable drug release over three months with ISL-triphosphate (ISL-TP) concentr54ations in PBMCs above levels predicted to be efficacious for PrEP. Similarly, ENG implants demonstrated sustained drug release with median [range] plasma ENG levels of 495 [229-1110] pg/mL, which suppressed progesterone within two weeks and showed no evidence of altering ISL pharmacokinetics. Two of the six ISL-98 mg implants broke during the study and induced implant-site reactions, whereas no reactions were observed with intact implants. We show that ISL and ENG biodegradable implants are safe and yield sufficient drug levels to achieve prevention targets. The evaluation of optimized implants with increased mechanical robustness is underway for improved durability and vaginal efficacy in a SHIV challenge model.
Keywords: HIV pre-exposure prophylaxis; biodegradable implant; etonogestrel; islatravir; multipurpose prevention technologies.
Conflict of interest statement
The authors (L.M.J., A.v.d.S., L.L., A.K. and E.H.L.) are inventors on pending patent applications filed by RTI. Author Ariane van der Straten is employed by the company ASTRA Consulting. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- UNAIDS Gender Every Minute. [(accessed on 20 June 2023)]. Available online: https://www.unaids.org/sites/default/files/20120607_infographic_gender-e....
-
- Wulandari L.P.L., He S.Y., Fairley C.K., Bavinton B.R., Marie-Schmidt H., Wiseman V., Guy R., Tang W., Zhang L., Ong J.J. Preferences for pre-exposure prophylaxis for HIV: A systematic review of discrete choice experiments. EClinicalMedicine. 2022;51:101507. doi: 10.1016/j.eclinm.2022.101507. - DOI - PMC - PubMed
-
- Quaife M., Eakle R., Cabrera Escobar M.A., Vickerman P., Kilbourne-Brook M., Mvundura M., Delany-Moretlwe S., Terris-Prestholt F. Divergent Preferences for HIV Prevention: A Discrete Choice Experiment for Multipurpose HIV Prevention Products in South Africa. Med. Decis. Mak. 2018;38:120–133. doi: 10.1177/0272989X17729376. - DOI - PubMed
-
- Browne E.N., Montgomery E.T., Mansfield C., Boeri M., Mange B., Beksinska M., Schwartz J.L., Clark M.R., Doncel G.F., Smit J., et al. Efficacy is Not Everything: Eliciting Women’s Preferences for a Vaginal HIV Prevention Product Using a Discrete-Choice Experiment. AIDS Behav. 2020;24:1443–1451. doi: 10.1007/s10461-019-02715-1. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

