Humoral Response Kinetics and Cross-Immunity in Hospitalized Patients with SARS-CoV-2 WT, Delta, or Omicron Infections: A Comparison between Vaccinated and Unvaccinated Cohorts
- PMID: 38140207
- PMCID: PMC10747008
- DOI: 10.3390/vaccines11121803
Humoral Response Kinetics and Cross-Immunity in Hospitalized Patients with SARS-CoV-2 WT, Delta, or Omicron Infections: A Comparison between Vaccinated and Unvaccinated Cohorts
Abstract
With the ongoing evolution of severe acute respiratory virus-2 (SARS-CoV-2), the number of confirmed COVID-19 cases continues to rise. This study aims to investigate the impact of vaccination status, SARS-CoV-2 variants, and disease severity on the humoral immune response, including cross-neutralizing activity, in hospitalized COVID-19 patients. This retrospective cohort study involved 122 symptomatic COVID-19 patients hospitalized in a single center. Patients were categorized based on the causative specific SARS-CoV-2 variants (33 wild-type (WT), 54 Delta and 35 Omicron) and their vaccination history. Sequential samples were collected to assess binding antibody responses (anti-S/RBD and anti-N) and surrogate virus neutralization tests (sVNTs) against WT, Omicron BA.1, and BA.4/5. The vaccinated breakthrough infection group (V) exhibited higher levels of anti-S/RBD compared to the variant-matched unvaccinated groups (UVs). The Delta infection resulted in a more rapid production of anti-S/RBD levels compared to infections with WT or Omicron variants. Unvaccinated severe WT or Delta infections had higher anti-S/RBD levels compared to mild cases, but this was not the case with Omicron infection. In vaccinated patients, there was no difference in antibody levels between mild and severe infections. Both Delta (V) and Omicron (V) groups showed strong cross-neutralizing activity against WT and Omicron (BA.1 and BA.4/5), ranging from 79.3% to 97.0%. WT (UV) and Delta (UV) infections had reduced neutralizing activity against BA.1 (0.8% to 12.0%) and BA.4/5 (32.8% to 41.0%). Interestingly, patients who received vaccines based on the ancestral spike exhibited positive neutralizing activity against BA.4/5, even though none of the study participants had been exposed to BA.4/5 and it is antigenically more advanced. Our findings suggest that a previous vaccination enhanced the humoral immune response and broadened cross-neutralizing activity to SARS-CoV-2 variants in hospitalized COVID-19 patients.
Keywords: SARS-CoV-2; humoral immunity; neutralization; vaccine; variant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



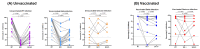
Similar articles
-
Comparison of Neutralizing Activity between Vaccinated and Unvaccinated Hospitalized COVID-19 Patients Infected with Delta, Omicron BA.1, or Omicron BA.2 Variant.Microorganisms. 2024 Mar 2;12(3):509. doi: 10.3390/microorganisms12030509. Microorganisms. 2024. PMID: 38543560 Free PMC article.
-
Pre-Omicron Vaccine Breakthrough Infection Induces Superior Cross-Neutralization against SARS-CoV-2 Omicron BA.1 Compared to Infection Alone.Int J Mol Sci. 2022 Jul 12;23(14):7675. doi: 10.3390/ijms23147675. Int J Mol Sci. 2022. PMID: 35887023 Free PMC article.
-
Immune responses in COVID-19 patients during breakthrough infection with SARS-CoV-2 variants Delta, Omicron-BA.1 and Omicron-BA.5.Front Immunol. 2023 Jul 13;14:1150667. doi: 10.3389/fimmu.2023.1150667. eCollection 2023. Front Immunol. 2023. PMID: 37520539 Free PMC article.
-
Emerging SARS-CoV-2 variants: Why, how, and what's next?Cell Insight. 2022 May 2;1(3):100029. doi: 10.1016/j.cellin.2022.100029. eCollection 2022 Jun. Cell Insight. 2022. PMID: 37193049 Free PMC article. Review.
-
Breakthrough COVID-19 Infections in the US: Implications for Prolonging the Pandemic.Vaccines (Basel). 2022 May 11;10(5):755. doi: 10.3390/vaccines10050755. Vaccines (Basel). 2022. PMID: 35632512 Free PMC article. Review.
References
-
- Lee J., Lee D.G., Jung J., Ryu J.H., Shin S., Cho S.Y., Lee R., Oh E.J. Comprehensive assessment of SARS-CoV-2 antibodies against various antigenic epitopes after naive COVID-19 infection and vaccination (BNT162b2 or ChAdOx1 nCoV-19) Front. Immunol. 2022;13:1038712. doi: 10.3389/fimmu.2022.1038712. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

