Prophylactic Vaccination and Intratumoral Boost with HER2-Expressing Oncolytic Herpes Simplex Virus Induces Robust and Persistent Immune Response against HER2-Positive Tumor Cells
- PMID: 38140209
- PMCID: PMC10747554
- DOI: 10.3390/vaccines11121805
Prophylactic Vaccination and Intratumoral Boost with HER2-Expressing Oncolytic Herpes Simplex Virus Induces Robust and Persistent Immune Response against HER2-Positive Tumor Cells
Abstract
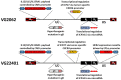
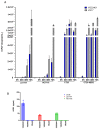
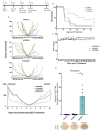
The development of effective cancer vaccines remains a significant challenge due to immune tolerance and limited clinical benefits. Oncolytic herpes simplex virus type 1 (oHSV-1) has shown promise as a cancer therapy, but efficacy is often limited in advanced cancers. In this study, we constructed and characterized a novel oHSV-1 virus (VG22401) expressing the human epidermal growth factor receptor 2 (HER2), a transmembrane glycoprotein overexpressed in many carcinomas. VG22401 exhibited efficient replication and HER2 payload expression in both human and mouse colorectal cancer cells. Mice immunized with VG22401 showed significant binding of serum anti-HER2 antibodies to HER2-expressing tumor cells, inducing antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). Furthermore, mice primed with VG22401 and intratumorally boosted with the same virus showed enhanced antitumor efficacy in a bilateral syngeneic HER2(+) tumor model, compared to HER2-null backbone virus. This effect was accompanied by the induction of anti-HER2 T cell responses. Our findings suggest that peripheral priming with HER2-expressing oHSV-1 followed by an intratumoral boost with the same virus can significantly enhance antitumor immunity and efficacy, presenting a promising strategy for cancer immunotherapy.
Keywords: HER2; cancer vaccine; herpes simplex virus; oncolytic virus.
Conflict of interest statement
All authors are current employees of Virogin Biotech Canada Ltd., have an ownership interest (including stock, patents, etc.) in Virogin Biotech Canada Ltd. This study was wholly funded by Virogin Biotech Canada Ltd., and patent applications have been filed to cover the technologies detailed herein.
Figures


Similar articles
-
CD40L-armed oncolytic herpes simplex virus suppresses pancreatic ductal adenocarcinoma by facilitating the tumor microenvironment favorable to cytotoxic T cell response in the syngeneic mouse model.J Immunother Cancer. 2022 Jan;10(1):e003809. doi: 10.1136/jitc-2021-003809. J Immunother Cancer. 2022. PMID: 35086948 Free PMC article.
-
IL-12 Expressing oncolytic herpes simplex virus promotes anti-tumor activity and immunologic control of metastatic ovarian cancer in mice.J Ovarian Res. 2016 Oct 27;9(1):70. doi: 10.1186/s13048-016-0282-3. J Ovarian Res. 2016. PMID: 27784340 Free PMC article.
-
Genotype of Immunologically Hot or Cold Tumors Determines the Antitumor Immune Response and Efficacy by Fully Virulent Retargeted oHSV.Viruses. 2021 Sep 1;13(9):1747. doi: 10.3390/v13091747. Viruses. 2021. PMID: 34578328 Free PMC article.
-
Modulation of the Intratumoral Immune Landscape by Oncolytic Herpes Simplex Virus Virotherapy.Front Oncol. 2017 Jun 26;7:136. doi: 10.3389/fonc.2017.00136. eCollection 2017. Front Oncol. 2017. PMID: 28695111 Free PMC article. Review.
-
Oncolytic herpes simplex virus-based strategies: toward a breakthrough in glioblastoma therapy.Front Microbiol. 2014 Jun 20;5:303. doi: 10.3389/fmicb.2014.00303. eCollection 2014. Front Microbiol. 2014. PMID: 24999342 Free PMC article. Review.
Cited by
-
Phase 1, open-label, multicenter, dose escalation safety and tolerability study of oncolytic virus OVV-01 in advanced solid tumors.J Immunother Cancer. 2025 Jun 5;13(6):e011517. doi: 10.1136/jitc-2025-011517. J Immunother Cancer. 2025. PMID: 40480657 Free PMC article. Clinical Trial.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

