High Protein Oral Nutritional Supplements Enable the Majority of Cancer Patients to Meet Protein Intake Recommendations during Systemic Anti-Cancer Treatment: A Randomised Controlled Parallel-Group Study
- PMID: 38140289
- PMCID: PMC10745925
- DOI: 10.3390/nu15245030
High Protein Oral Nutritional Supplements Enable the Majority of Cancer Patients to Meet Protein Intake Recommendations during Systemic Anti-Cancer Treatment: A Randomised Controlled Parallel-Group Study
Abstract
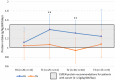
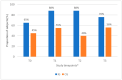
ESPEN guidelines recommend a minimum protein intake of 1.0 g/kg body weight (BW) per day to maintain or restore lean body mass in patients with cancer. During anti-cancer treatment, optimal protein intake is difficult to achieve. We investigated whether a high-protein, low-volume oral nutritional supplement (ONS) supports patients in meeting recommendations. A multi-centre, randomised, controlled, open-label, parallel-group study was carried out in nine hospitals (five countries) between January 2019 and July 2021 in colorectal and lung cancer patients undergoing first-line systemic treatment with chemo(radio-) or immunotherapy. Subjects were randomised (2:1) to receive Fortimel Compact Protein® or standard care. Protein intake was assessed with a 3-day food diary (primary outcome). BW was a secondary outcome. Due to challenges in recruitment, the study was terminated prematurely with 42 patients randomised (intervention group (IG) 28; control group (CG) 14). At T1 and T2, protein intake was statistically significantly higher in the IG compared to the CG (1.40 vs. 1.07 g/kg/day at T1, p = 0.008; 1.32 vs. 0.94 g/kg/day at T2, p = 0.002). At baseline, only 65% (IG) and 45% (CG) of patients met ESPEN minimum protein intake recommendations. However, at T1 and T2 in the IG, a higher proportion of patients met recommendations than in the CG (88% vs. 55% and 40%). No statistically significant difference between study groups was observed for BW. Mean compliance to the ONS was 73.4%. A high-protein, low-volume ONS consumed twice daily enables the majority of patients to reach minimal ESPEN protein recommendations.
Keywords: cancer; high protein; malnutrition; nutrition support; oral nutritional supplements.
Conflict of interest statement
N.v.W., F.S, M.Y.-E.S., E.B., W.L. and S.K. declare no conflict of interest. A.-M.D. has (to her institution) received grants from or has contracts with AMGEN, the Dutch Cancer Society and HANART. A.-M.D. has (to her institution) received consulting fees from AMGEN, Bayer, Boehringer Ingelheim, Sanofy, Roche, Jansen and Astra Zeneca. A.-M.D. has participated on a Data Safety Monitoring Board or Advisory Board for Takeda and Roche. A.-M.D. has held the position of Chair (unpaid) for the European Organisation for Research and Treatment of Cancer Lung Cancer Group. M.V. and C.A.v.d.B. are employed by Danone Nutricia Research. The funders contributed to the design of the study; in the collection, analyses and interpretation of data; in the writing of the manuscript; and in the decision to publish the results.
Figures

References
-
- Zhang X., Tang M., Zhang Q., Zhang K.P., Guo Z.Q., Xu H.X., Yuan K.T., Yu M., Braga M., Cederholm T., et al. The GLIM criteria as an effective tool for nutrition assessment and survival prediction in older adult cancer patients. Clin. Nutr. 2021;40:1224–1232. doi: 10.1016/j.clnu.2020.08.004. - DOI - PubMed
-
- Muscaritoli M., Lucia S., Farcomeni A., Lorusso V., Saracino V., Barone C., Plastino F., Gori S., Magarotto R., Carteni G., et al. Prevalence of malnutrition in patients at first medical oncology visit: The PreMiO study. Oncotarget. 2017;8:79884–79896. doi: 10.18632/oncotarget.20168. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

