Nutritional Modulation of Hepcidin in the Treatment of Various Anemic States
- PMID: 38140340
- PMCID: PMC10745534
- DOI: 10.3390/nu15245081
Nutritional Modulation of Hepcidin in the Treatment of Various Anemic States
Abstract
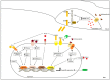
Twenty years after its discovery, hepcidin is still considered the main regulator of iron homeostasis in humans. The increase in hepcidin expression drastically blocks the flow of iron, which can come from one's diet, from iron stores, and from erythrophagocytosis. Many anemic conditions are caused by non-physiologic increases in hepcidin. The sequestration of iron in the intestine and in other tissues poses worrying premises in view of discoveries about the mechanisms of ferroptosis. The nutritional treatment of these anemic states cannot ignore the nutritional modulation of hepcidin, in addition to the bioavailability of iron. This work aims to describe and summarize the few findings about the role of hepcidin in anemic diseases and ferroptosis, as well as the modulation of hepcidin levels by diet and nutrients.
Keywords: anemia; ferroptosis; hepcidin; iron homeostasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Troutt J.S., Rudling M., Persson L., Ståhle L., Angelin B., Butterfield A.M., Schade A.E., Cao G., Konrad R.J. Circulating human hepcidin-25 concentrations display a diurnal rhythm, increase with prolonged fasting, and are reduced by growth hormone administration. Clin. Chem. 2012;58:1225–1232. doi: 10.1373/clinchem.2012.186866. - DOI - PubMed
-
- McKie A.T., Marciani P., Rolfs A., Brennan K., Wehr K., Barrow D., Miret S., Bomford A., Peters T.J., Farzaneh F., et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol. Cell. 2000;5:299–309. doi: 10.1016/S1097-2765(00)80425-6. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

