Antiviral Potential of Azelastine against Major Respiratory Viruses
- PMID: 38140540
- PMCID: PMC10747764
- DOI: 10.3390/v15122300
Antiviral Potential of Azelastine against Major Respiratory Viruses
Abstract
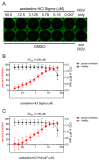
The Coronavirus Disease 2019 (COVID-19) pandemic and the subsequent increase in respiratory viral infections highlight the need for broad-spectrum antivirals to enable a quick and efficient reaction to current and emerging viral outbreaks. We previously demonstrated that the antihistamine azelastine hydrochloride (azelastine-HCl) exhibited in vitro antiviral activity against SARS-CoV-2. Furthermore, in a phase 2 clinical study, a commercial azelastine-containing nasal spray significantly reduced the viral load in SARS-CoV-2-infected individuals. Here, we evaluate the efficacy of azelastine-HCl against additional human coronaviruses, including the SARS-CoV-2 omicron variant and a seasonal human coronavirus, 229E, through in vitro infection assays, with azelastine showing a comparable potency against both. Furthermore, we determined that azelastine-HCl also inhibits the replication of Respiratory syncytial virus A (RSV A) in both prophylactic and therapeutic settings. In a human 3D nasal tissue model (MucilAirTM-Pool, Epithelix), azelastine-HCl protected tissue integrity and function from the effects of infection with influenza A H1N1 and resulted in a reduced viral load soon after infection. Our results suggest that azelastine-HCl has a broad antiviral effect and can be considered a safe option against the most common respiratory viruses to prevent or treat such infections locally in the form of a nasal spray that is commonly available globally.
Keywords: RSV; antiviral; azelastine; drug repurposing; respiratory viruses.
Conflict of interest statement
G.N., E.N. and V.S. are employees of CEBINA GmbH, K.F., J.A. and N.K. were employees of CEBINA GmbH at the time of this study.
Figures


References
-
- Wang X., Li Y., Mei X., Bushe E., Campbell H., Nair H. Global Hospital Admissions and In-Hospital Mortality Associated with All-cause and virus-Specific acute Lower Respiratory Infections in Children and Adolescents Aged 5–19 Years between 1995 and 2019: A Systematic Review and Modelling Study. BMJ Glob. Health. 2021;6:6014. doi: 10.1136/bmjgh-2021-006014. - DOI - PMC - PubMed
-
- GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the Global, Regional, and National Morbidity, Mortality, and Aetiologies of Lower Respiratory Infections in 195 Countries, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018;18:1191–1210. doi: 10.1016/S1473-3099(18)30310-4. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

