Discovery and Characterization of IFITM S-Palmitoylation
- PMID: 38140570
- PMCID: PMC10747768
- DOI: 10.3390/v15122329
Discovery and Characterization of IFITM S-Palmitoylation
Abstract
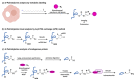
Interferon-induced transmembrane proteins (IFITM1, 2 and 3) are important host antiviral defense factors. They are active against viruses like the influenza A virus (IAV), dengue virus (DENV), Ebola virus (EBOV), Zika virus (ZIKV) and severe acute respiratory syndrome coronavirus (SARS-CoV). In this review, we focus on IFITM3 S-palmitoylation, a reversible lipid modification, and describe its role in modulating IFITM3 antiviral activity. Our laboratory discovered S-palmitoylation of IFITMs using chemical proteomics and demonstrated the importance of highly conserved fatty acid-modified Cys residues in IFITM3 antiviral activity. Further studies showed that site-specific S-palmitoylation at Cys72 is important for IFITM3 trafficking to restricted viruses (IAV and EBOV) and membrane-sterol interactions. Thus, site-specific lipid modification of IFITM3 directly regulates its antiviral activity, cellular trafficking, and membrane-lipid interactions.
Keywords: IFITM3; S-palmitoylation; influenza A virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

