Cholesterol Metabolism in Antigen-Presenting Cells and HIV-1 Trans-Infection of CD4+ T Cells
- PMID: 38140588
- PMCID: PMC10747884
- DOI: 10.3390/v15122347
Cholesterol Metabolism in Antigen-Presenting Cells and HIV-1 Trans-Infection of CD4+ T Cells
Abstract
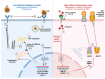
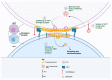
Antiretroviral therapy (ART) provides an effective method for managing HIV-1 infection and preventing the onset of AIDS; however, it is ineffective against the reservoir of latent HIV-1 that persists predominantly in resting CD4+ T cells. Understanding the mechanisms that facilitate the persistence of the latent reservoir is key to developing an effective cure for HIV-1. Of particular importance in the establishment and maintenance of the latent viral reservoir is the intercellular transfer of HIV-1 from professional antigen-presenting cells (APCs-monocytes/macrophages, myeloid dendritic cells, and B lymphocytes) to CD4+ T cells, termed trans-infection. Whereas virus-to-cell HIV-1 cis infection is sensitive to ART, trans-infection is impervious to antiviral therapy. APCs from HIV-1-positive non-progressors (NPs) who control their HIV-1 infection in the absence of ART do not trans-infect CD4+ T cells. In this review, we focus on this unique property of NPs that we propose is driven by a genetically inherited, altered cholesterol metabolism in their APCs. We focus on cellular cholesterol homeostasis and the role of cholesterol metabolism in HIV-1 trans-infection, and notably, the link between cholesterol efflux and HIV-1 trans-infection in NPs.
Keywords: HIV-1; antigen-presenting cells; antiretroviral therapy; cholesterol metabolism; immunometabolism; latent reservoir; non-progressor; trans-infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Grant R.M., Lama J.R., Anderson P.L., McMahan V., Liu A.Y., Vargas L., Goicochea P., Casapía M., Guanira-Carranza J.V., Ramirez-Cardich M.E., et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

