Smoking, Alcohol Intake and Torque Teno Virus in Stable Kidney Transplant Recipients
- PMID: 38140628
- PMCID: PMC10748022
- DOI: 10.3390/v15122387
Smoking, Alcohol Intake and Torque Teno Virus in Stable Kidney Transplant Recipients
Abstract
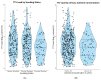
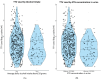
Torque Teno Virus (TTV) is a non-pathogenic virus that is highly prevalent among kidney transplant recipients (KTRs). Its circulating load is associated with an immunological status in KTR and is considered a promising tool for guiding immunosuppression. To allow for optimal guidance, it is important to identify other determinants of TTV load. We aimed to investigate the potential association of smoking and alcohol intake with TTV load. For this cross-sectional study, serum TTV load was measured using PCR in stable kidney transplant recipients at ≥1 year after transplantation, and smoking status and alcohol intake were assessed through questionnaires and measurements of urinary cotinine and ethyl glucuronide. A total of 666 KTRs were included (57% male). A total of 549 KTR (82%) had a detectable TTV load (3.1 ± 1.5 log10 copies/mL). In KTR with a detectable TTV load, cyclosporin and tacrolimus use were positively associated with TTV load (St. β = 0.46, p < 0.001 and St. β = 0.66, p < 0.001, respectively), independently of adjustment for potential confounders. Current smoking and alcohol intake of >20 g/day were negatively associated with TTV load (St. β = -0.40, p = 0.004 and St. β = -0.33, p = 0.009, respectively), independently of each other and of adjustment for age, sex, kidney function, time since transplantation and calcineurin inhibitor use. This strong association of smoking and alcohol intake with TTV suggests a need to account for the smoking status and alcohol intake when applying TTV guided immunosuppression in KTR.
Keywords: Torque Teno Virus; alcohol; immunosuppression; kidney transplantation; smoking.
Conflict of interest statement
The Nephrology department of the University Medical Center Groningen is one of the trial sites of the TTV GUIDE IT study, coordinated by the Medical University of Vienna.
Figures
References
-
- Andrews L.M., Li Y., De Winter B.C.M., Shi Y.Y., Baan C.C., Van Gelder T., Hesselink D.A. Pharmacokinetic Considerations Related to Therapeutic Drug Monitoring of Tacrolimus in Kidney Transplant Patients. Expert Opin. Drug Metab. Toxicol. 2017;13:1225–1236. doi: 10.1080/17425255.2017.1395413. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

