Restoration of a paraventricular thalamo-accumbal behavioral suppression circuit prevents reinstatement of heroin seeking
- PMID: 38141605
- PMCID: PMC10939883
- DOI: 10.1016/j.neuron.2023.11.024
Restoration of a paraventricular thalamo-accumbal behavioral suppression circuit prevents reinstatement of heroin seeking
Abstract
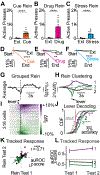
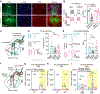
Lack of behavioral suppression typifies substance use disorders, yet the neural circuit underpinnings of drug-induced behavioral disinhibition remain unclear. Here, we employ deep-brain two-photon calcium imaging in heroin self-administering mice, longitudinally tracking adaptations within a paraventricular thalamus to nucleus accumbens behavioral inhibition circuit from the onset of heroin use to reinstatement. We find that select thalamo-accumbal neuronal ensembles become profoundly hypoactive across the development of heroin seeking and use. Electrophysiological experiments further reveal persistent adaptations at thalamo-accumbal parvalbumin interneuronal synapses, whereas functional rescue of these synapses prevents multiple triggers from initiating reinstatement of heroin seeking. Finally, we find an enrichment of μ-opioid receptors in output- and cell-type-specific paraventricular thalamic neurons, which provide a mechanism for heroin-induced synaptic plasticity and behavioral disinhibition. These findings reveal key circuit adaptations that underlie behavioral disinhibition in opioid dependence and further suggest that recovery of this system would reduce relapse susceptibility.
Keywords: addiction; behavioral disinhibition; multiphoton calcium imaging; opioid use disorder; paraventricular thalamus; parvalbumin interneurons.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors have no competing interests to declare.
Figures





References
-
- Webster LR (2017). Risk Factors for Opioid-Use Disorder and Overdose. Anesth. Analg 125. - PubMed
MeSH terms
Substances
Grants and funding
- T32 AA007474/AA/NIAAA NIH HHS/United States
- F32 DA053830/DA/NIDA NIH HHS/United States
- R01 DA054271/DA/NIDA NIH HHS/United States
- T32 DA053558/DA/NIDA NIH HHS/United States
- I01 BX006179/BX/BLRD VA/United States
- TL1 TR001451/TR/NCATS NIH HHS/United States
- R01 AA030796/AA/NIAAA NIH HHS/United States
- R01 DA049711/DA/NIDA NIH HHS/United States
- UL1 TR001450/TR/NCATS NIH HHS/United States
- P50 DA046373/DA/NIDA NIH HHS/United States
- T32 DA007288/DA/NIDA NIH HHS/United States
- K99 DA058049/DA/NIDA NIH HHS/United States
- R01 DA054154/DA/NIDA NIH HHS/United States
- R25 GM113278/GM/NIGMS NIH HHS/United States
- F32 DA057794/DA/NIDA NIH HHS/United States
- F31 DA052186/DA/NIDA NIH HHS/United States
- R01 DA051650/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

