Allelochemical root-growth inhibitors in low-molecular-weight cress-seed exudate
- PMID: 38141653
- PMCID: PMC11006535
- DOI: 10.1093/aob/mcad200
Allelochemical root-growth inhibitors in low-molecular-weight cress-seed exudate
Abstract
Background and aims: Cress seeds release allelochemicals that over-stimulate the elongation of hypocotyls of neighbouring (potentially competing) seedlings and inhibit their root growth. The hypocotyl promoter is potassium, but the root inhibitor was unidentified; its nature is investigated here.
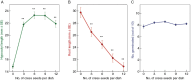
Methods: Low-molecular-weight cress-seed exudate (LCSE) from imbibed Lepidium sativum seeds was fractionated by phase partitioning, paper chromatography, high-voltage electrophoresis and gel-permeation chromatography (on Bio-Gel P-2). Fractions, compared with pure potassium salts, were bioassayed for effects on Amaranthus caudatus seedling growth in the dark for 4 days.
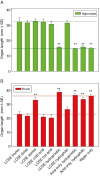
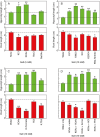
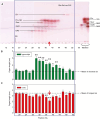
Key results: The LCSE robustly promoted amaranth hypocotyl elongation and inhibited root growth. The hypocotyl inhibitor was non-volatile, hot acid stable, hydrophilic and resistant to incineration, as expected for K+. The root inhibitor(s) had similar properties but were organic (activity lost on incineration). The root inhibitor(s) remained in the aqueous phase (at pH 2.0, 6.5 and 9.0) when partitioned against butan-1-ol or toluene, and were thus hydrophilic. Activity was diminished after electrophoresis, but the remaining root inhibitors were neutral. They became undetectable after paper chromatography; therefore, they probably comprised multiple compounds, which separated from each other, in part, during fractionation. On gel-permeation chromatography, the root inhibitor co-eluted with hexoses.
Conclusions: Cress-seed allelochemicals inhibiting root growth are different from the agent (K+) that over-stimulates hypocotyl elongation and the former probably comprise a mixture of small, non-volatile, hydrophilic, organic substances. Abundant components identified chromatographically and by electrophoresis in cress-seed exudate fitting this description include glucose, fructose, sucrose and galacturonic acid. However, none of these sugars co-chromatographed and co-electrophoresed with the root-inhibitory principle of LCSE, and none of them (in pure form at naturally occurring concentrations) inhibited root growth. We conclude that the root-inhibiting allelochemicals of cress-seed exudate remain unidentified.
Keywords: Allelochemicals; amaranth (Amaranthus caudatus); bioassay; chromatography; cress (Lepidium sativum); electrophoresis; hypocotyl elongation; potassium salts; root growth; seed exudate.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Annals of Botany Company. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Asaduzzaman M, An M, Pratley JE, Luckett DJ, Lemerle D, Coombes N.. 2016. The seedling root response of annual ryegrass (Lolium rigidum) to neighbouring seedlings of a highly-allelopathic canola (Brassica napus). Flora 219: 18–24.
-
- Boydston RA, Morra MJ, Borek V, Clayton L, Vaughn SF.. 2011. Onion and weed response to mustard (Sinapis alba) seed meal. Weed Science 59: 546–552.
-
- Cordeau S, Triolet M, Wayman S, Steinberg C, Guillemin J-P.. 2016. Bioherbicides: dead in the water? A review of the existing products for integrated weed management. Crop Protection 87: 44–49.
-
- Cornes D. 2005. Callisto: a very successful maize herbicide inspired by allelochemistry. Proceedings of 4th World Congress on Allelopathy, Wagga Wagga, NSW, Australia. http://www.regional.org.au/au/allelopathy/2005/2/7/2636_cornesd.htm
-
- Curl EA, Truelove B.. 1986. The rhizosphere. New York: Springer.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

