Type 1 VWD classification revisited: novel insights from combined analysis of the LoVIC and WiN studies
- PMID: 38142407
- PMCID: PMC11033584
- DOI: 10.1182/blood.2023022457
Type 1 VWD classification revisited: novel insights from combined analysis of the LoVIC and WiN studies
Abstract
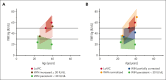
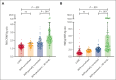
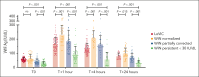
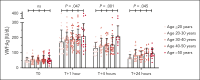
There is significant ongoing debate regarding type 1 von Willebrand disease (VWD) defintion. Previous guidelines recommended patients with von Willebrand factor (VWF) levels <30 IU/dL be diagnosed type 1 VWD, whereas patients with significant bleeding and VWF levels from 30 to 50 IU/dL be diagnosed with low VWF. To elucidate the relationship between type 1 VWD and low VWF in the context of age-induced increases in VWF levels, we combined data sets from 2 national cohort studies: 162 patients with low VWF from the Low VWF in Ireland Cohort (LoVIC) and 403 patients with type 1 VWD from the Willebrand in The Netherlands (WiN) studies. In 47% of type 1 VWD participants, VWF levels remained <30 IU/dL despite increasing age. Conversely, VWF levels increased to the low VWF range (30-50 IU/dL) in 30% and normalized (>50 IU/dL) in 23% of type 1 VWD cases. Crucially, absolute VWF antigen (VWF:Ag) levels and increase of VWF:Ag per year overlapped between low VWF and normalized type 1 VWD participants. Moreover, multiple regression analysis demonstrated that VWF:Ag levels in low VWF and normalized type 1 VWD patients would not have been different had they been diagnosed at the same age (β = 0.00; 95% confidence interval, -0.03 to 0.04). Consistently, no difference was found in the prevalence of VWF sequence variants; factor VIII activity/VWF:Ag or VWF propeptide/VWF:Ag ratios; or desmopressin responses between low VWF and normalized type 1 VWD patients. In conclusion, our findings demonstrate that low VWF does not constitute a discrete clinical or pathological entity. Rather, it is part of an age-dependent type 1 VWD evolving phenotype. Collectively, these data have important implications for future VWD classification criteria.
© 2024 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: F.A. received research support from CSL Behring, Takeda, Octapharma, and Sobi; and also received travel grants from Sobi. F.W.G.L. has received unrestricted grants/research funding from CSL Behring, uniQure, Sobi, and Takeda; consultancy fees from BioMarin, CSL Behring, Takeda, and uniQure (all fees to the institution); and served as a data safety and monitoring board member for a study sponsored by Roche. J.S.O. has served on the speaker’s bureau for Baxter, Bayer, Novo Nordisk, Sobi, Boehringer Ingelheim, Leo Pharma, Takeda, and Octapharma; served on the advisory boards of Baxter, Sobi, Bayer, Octapharma CSL Behring, Daiichi Sankyo, Boehringer Ingelheim, Takeda, and Pfizer; and has also received research grant funding awards from 3M, Baxter, Bayer, Pfizer, Shire, Takeda, 3M, and Novo Nordisk. D.D. has received honoraria from Takeda and educational support sponsorship from NovoNordisk and Amgen. M.L. has received consultancy fees from Sobi, Band Therapeutics, and CSL Behring; honoraria from CSL Behring and Pfizer; and indirect funding for development of educational content from Takeda. J.G.v.d.B. received research funding from Novo Nordisk. N.M.O. served on advisory boards for Sobi, F. Hoffman-La Roche Ltd, uniQure, CSL Behring, AstraZeneca, and Freeline and on the speaker’s bureau for Novo Nordisk, Sobi, CSL Behring, Bayer, and Takeda. S.E.M.S. has received research funding from Bayer. R.I.B.’s institution has received research support/clinical trial funding from Bayer, Takeda, Pfizer, Daiichi Sankyo, CSL Behring, Roche, Amgen, AstraZeneca, AbbVie, Sanofi, Acerta Pharma, Jansen-Cileg, Bristol Myers Squibb, Boehringer Ingelheim, Werfen, and Technoclone, unrelated to this study. K.M. reports speaker fees from Alexion, Bayer, and CSL Behring; participation in trial steering committees for Bayer and AstraZeneca; consulting fees from uniQure and Therini; and participation in data monitoring and end point adjudication committee for Octapharma (all fees paid to the institution). P.J. receives research funding from Bayer and consultancy fees from Band/Guardian Therapeutics, Star/Vega Therapeutics, and Roche. K.F. has received unrestricted grants/research funding from CSL Behring, Sobi, and Takeda for research unrelated to this study and consultancy fees from SOBI, Sanofi, Takeda, Novo Nordisk, and Roche (all fees to the institution). K.P.M.v.G. has received an unrestricted research grant from Octapharma. The remaining authors declare no competing financial interests.
Figures


Comment in
-
An evolving understanding of low VWF and type 1 VWD.Blood. 2024 Apr 4;143(14):1324-1326. doi: 10.1182/blood.2023023488. Blood. 2024. PMID: 38573605 No abstract available.
References
-
- Leebeek FW, Eikenboom JC. Von Willebrand's disease. N Engl J Med. 2016;375(21):2067–2080. - PubMed
-
- Nichols WL, Hultin MB, James AH, et al. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA) Haemophilia. 2008;14(2):171–232. - PubMed
-
- Sadler JE, Budde U, Eikenboom JC, et al. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J Thromb Haemost. 2006;4(10):2103–2114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

