Resistant and refractory migraine: clinical presentation, pathophysiology, and management
- PMID: 38142636
- PMCID: PMC10788408
- DOI: 10.1016/j.ebiom.2023.104943
Resistant and refractory migraine: clinical presentation, pathophysiology, and management
Abstract
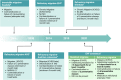
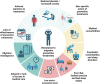
Migraine is a leading cause of disability worldwide. A minority of individuals with migraine develop resistant or refractory conditions characterised by ≥ 8 monthly days of debilitating headaches and inadequate response, intolerance, or contraindication to ≥3 or all preventive drug classes, respectively. Resistant and refractory migraine are emerging clinical definitions stemming from better knowledge of the pathophysiology of migraine and from the advent of migraine-specific preventive treatments. Resistant migraine mostly results from drug failures, while refractory migraine has complex and still unknown mechanisms that impair the efficacy of preventive treatments. Individuals with resistant migraine can be treated with migraine-specific preventive drugs. The management of refractory migraine is challenging and often unsuccessful, being based on combinations of different drugs and non-pharmacological treatment. Future research should aim to identify individuals at risk of developing treatment failures, prevent the condition, investigate the mechanisms of refractoriness to treatments, and find effective treatment strategies.
Keywords: CGRP; Migraine-related disability; Non-pharmacological treatments; Refractory migraine; Resistant migraine.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests RO reports consulting fees from Teva, direct payments from Teva, Eli Lilly, Novartis, AbbVie, and Pfizer, support for attending meetings and/or travel from Novartis and Teva, participation to advisory boards from Eli Lilly, and other support from Novartis and Allergan-AbbVie; he is a Junior Editorial Board member of The Journal of Headache and Pain. SS reports grants from Novartis and Uriach, consulting fees from Abbott, Allergan, AbbVie, Novartis, Teva, Eli Lilly, Pfizer, Lundbeck, Novo Nordisk, and AstraZeneca, direct payment from Abbott, Allergan-AbbVie, Novartis, Teva, Eli Lilly, Pfizer, Lundbeck, Novo Nordisk, and AstraZeneca, support for attending meetings and/or travel from Eli Lilly, Novartis, Teva, and Lundbeck, receipt of equipment from Allergan-AbbVie and Novo Nordisk; she is President Elect of the European Stroke Organisation and Editor-in-Chief of Cephalalgia. APA received grants or contracts from Brain Research UK, Medical Research Council, Medical Research Foundation, Rosetrees, and Migraine Trust, consulting fees from Eli Lilly and AbbVie, direct payment from Eli Lilly, AbbVie, eNeura, Autonomic Technologies, and Novartis, participation to advisory boards from Eli Lilly and AbbVie; she is Chair of the Communications Committee of the International Headache Society and Chair of the Headache SIG of the British Pain Society. TPJ reports grants from the European Regional Development Funds (ERDF), Innovation Fund of the Federal Joint Committee (Germany) and Novartis; compensation from Allergan, Abbvie, Chordate, Grünenthal, Hormosan, Lilly, Lundbeck, Novartis, Pfizer, Teva and Sanofi for consultant services and/or speaker honoraria; he is President of the German Migraine and Headache Society and member of the Educational Committee of the German Pain Society. EDM and MTM report no conflict of interest.
Figures


References
-
- Goadsby P.J., Schoenen J., Ferrari M.D., Silberstein S.D., Dodick D. Towards a definition of intractable headache for use in clinical practice and trials. Cephalalgia. 2006;26(9):1168–1170. - PubMed
-
- Schulman E.A., Lake A.E., 3rd, Goadsby P.J., et al. Defining refractory migraine and refractory chronic migraine: proposed criteria from the refractory headache special interest section of the American Headache society. Headache. 2008;48(6):778–782. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

