Antiviral potency of long-acting islatravir subdermal implant in SHIV-infected macaques
- PMID: 38142963
- PMCID: PMC10922355
- DOI: 10.1016/j.jconrel.2023.12.031
Antiviral potency of long-acting islatravir subdermal implant in SHIV-infected macaques
Abstract
Treatment nonadherence is a pressing issue in people living with HIV (PLWH), as they require lifelong therapy to maintain viral suppression. Poor adherence leads to antiretroviral (ARV) resistance, transmission to others, AIDS progression, and increased morbidity and mortality. Long-acting (LA) ARV therapy is a promising strategy to combat the clinical drawback of user-dependent dosing. Islatravir (ISL) is a promising candidate for HIV treatment given its long half-life and high potency. Here we show constant ISL release from a subdermal LA nanofluidic implant achieves viral load reduction in SHIV-infected macaques. Specifically, a mean delivery dosage of 0.21 ± 0.07 mg/kg/day yielded a mean viral load reduction of -2.30 ± 0.53 log10 copies/mL at week 2, compared to baseline. The antiviral potency of the ISL delivered from the nanofluidic implant was higher than oral ISL dosed either daily or weekly. At week 3, viral resistance to ISL emerged in 2 out of 8 macaques, attributable to M184V mutation, supporting the need of combining ISL with other ARV for HIV treatment. The ISL implant produced moderate reactivity in the surrounding tissue, indicating tolerability. Overall, we present the ISL subdermal implant as a promising approach for LA ARV treatment in PLWH.
Keywords: Drug delivery; HIV treatment; Implantable devices; Islatravir; Refillable.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
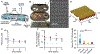
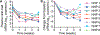

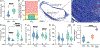

References
-
- Guaraldi G, Borghi V, Milic J, Carli F, Cuomo G, Menozzi M, Santoro A, Orlando G, Puzzolante C, Meschiari M, Franceschini E, Bedini A, Ferrari F, Gennari W, Sarti M, Mussini C, The Impact of COVID-19 on UNAIDS 90-90-90 Targets: Calls for New HIV Care Models, Open Forum Infectious Diseases, 8 (2021) ofab283. - PMC - PubMed
-
- Karaosmanoglu HK, How Does the Covid-19 Pandemic Affect the Target 90-90-90?, Curr HIV Res, 19 (2021) 103–105. - PubMed
-
- De Lay PR, Benzaken A, Karim QA, Aliyu S, Amole C, Ayala G, Chalkidou K, Chang J, Clayton M, Couto A, Dieffenbach C, Dybul M, El Sadr W, Gorgens M, Low-Beer D, Mesbah S, Saveedra J, Sirinirund P, Stover J, Syarif O, Taslim A, Thiam S, Njenga LW, Ghys PD, Izazola-Licea JA, Frescura L, Lamontagne E, Godfrey-Faussett P, Fontaine C, Semini I, Hader S, Ending AIDS as a public health threat by 2030: Time to reset targets for 2025, PLoS Med, 18 (2021) e1003649. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

