5-Flucytosine Longitudinal Antifungal Susceptibility Testing of Cryptococcus neoformans: A Substudy of the EnACT Trial Testing Oral Amphotericin
- PMID: 38143852
- PMCID: PMC10745249
- DOI: 10.1093/ofid/ofad596
5-Flucytosine Longitudinal Antifungal Susceptibility Testing of Cryptococcus neoformans: A Substudy of the EnACT Trial Testing Oral Amphotericin
Abstract
Background: The EnACT trial was a phase 2 randomized clinical trial conducted in Uganda, which evaluated a novel orally delivered lipid nanocrystal (LNC) amphotericin B in combination with flucytosine for the treatment of cryptococcal meningitis. When flucytosine (5FC) is used as monotherapy in cryptococcosis, 5FC can induce resistant Cryptococcus mutants. Oral amphotericin B uses a novel drug delivery mechanism, and we assessed whether resistance to 5FC develops during oral LNC-amphotericin B therapy.
Methods: We enrolled Ugandans with HIV diagnosed with cryptococcal meningitis and who were randomized to receive 5FC and either standard intravenous (IV) amphotericin B or oral LNC-amphotericin B. We used broth microdilution to measure the minimum inhibitory concentration (MIC) of the first and last cryptococcal isolates in each participant. Breakpoints are inferred from 5FC in Candida albicans. We measured cerebral spinal fluid (CSF) 5FC concentrations by liquid chromatography and tandem mass spectrometry.
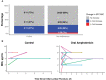
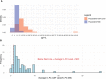
Results: Cryptococcus 5FC MIC50 was 4 µg/mL, and MIC90 was 8 µg/mL. After 2 weeks of therapy, there was no evidence of 5FC resistance developing, defined as a >4-fold change in susceptibility in any Cryptococcus isolate tested. The median CSF 5FC concentration to MIC ratio (interquartile range) was 3.0 (1.7-5.5) µg/mL. There was no association between 5FC/MIC ratio and early fungicidal activity of the quantitative rate of CSF yeast clearance (R2 = 0.004; P = .63).
Conclusions: There is no evidence of baseline resistance to 5FC or incident resistance during combination therapy with oral or IV amphotericin B in Uganda. Oral amphotericin B can safely be used in combination with 5FC.
Keywords: 5-flucytosine resistance; antifungal resistance; antifungal susceptibility testing; cryptococcal meningitis.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors: no reported conflicts.
Figures