Endoplasmic Reticulum Calcium Mediates Drosophila Wing Development
- PMID: 38143873
- PMCID: PMC10733776
- DOI: 10.1089/bioe.2022.0036
Endoplasmic Reticulum Calcium Mediates Drosophila Wing Development
Abstract
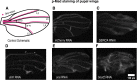
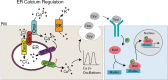
Background: The temporal dynamics of morphogen presentation impacts transcriptional responses and tissue patterning. However, the mechanisms controlling morphogen release are far from clear. We found that inwardly rectifying potassium (Irk) channels regulate endogenous transient increases in intracellular calcium and bone morphogenetic protein (BMP/Dpp) release for Drosophila wing development. Inhibition of Irk channels reduces BMP/Dpp signaling, and ultimately disrupts wing morphology. Ion channels impact development of several tissues and organisms in which BMP signaling is essential. In neurons and pancreatic beta cells, Irk channels modulate membrane potential to affect intracellular Ca++ to control secretion of neurotransmitters and insulin. Based on Irk activity in neurons, we hypothesized that electrical activity controls endoplasmic reticulum (ER) Ca++ release into the cytoplasm to regulate the release of BMP.
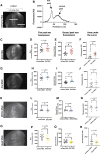
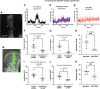
Materials and methods: To test this hypothesis, we reduced expression of four proteins that control ER calcium, Stromal interaction molecule 1 (Stim), Calcium release-activated calcium channel protein 1 (Orai), SarcoEndoplasmic Reticulum Calcium ATPase (SERCA), small conductance calcium-activated potassium channel (SK), and Bestrophin 2 (Best2) using RNAi and documented wing phenotypes. We use live imaging to study calcium and Dpp release within pupal wings and larval wing discs. Additionally, we employed immunohistochemistry to characterize Small Mothers Against Decapentaplegic (SMAD) phosphorylation downstream of the BMP/Dpp pathway following RNAi knockdown.
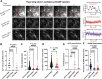
Results: We found that reduced Stim and SERCA function decreases amplitude and frequency of endogenous calcium transients in the wing disc and reduced BMP/Dpp release.
Conclusion: Our results suggest control of ER calcium homeostasis is required for BMP/Dpp release, and Drosophila wing development.
Keywords: BMP; Drosophila wing development; Orai; SERCA; Stim; bestrophin; bone morphogenic protein; calcium; endoplasmic reticulum; transients; wing venation.
Copyright 2023, Mary Ann Liebert, Inc., publishers.
Conflict of interest statement
No competing financial interests exist.
Figures


References
-
- Letsou A, Arora K, Wrana JL, et al. . Drosophila Dpp signaling is mediated by the punt gene product: A dual ligand-binding type II receptor of the TGF beta receptor family. Cell 1995;80(6):899–908. - PubMed
-
- Newfeld SJ, Mehra A, Singer MA, et al. . Mothers against Dpp participates in a DDP/TGF-beta responsive serine-threonine kinase signal transduction cascade. Development 1997;124(16):3167–3176. - PubMed
-
- Chen Y, Riese MJ, Killinger MA, et al. . A genetic screen for modifiers of Drosophila decapentaplegic signaling identifies mutations in punt, Mothers against Dpp and the BMP-7 homologue, 60A. Development 1998;125(9):1759–1768. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
