TRPA1 channel mediates methylglyoxal-induced mouse bladder dysfunction
- PMID: 38143915
- PMCID: PMC10739337
- DOI: 10.3389/fphys.2023.1308077
TRPA1 channel mediates methylglyoxal-induced mouse bladder dysfunction
Abstract
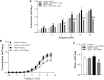
Introduction: The transient receptor potential ankyrin 1 channel (TRPA1) is expressed in urothelial cells and bladder nerve endings. Hyperglycemia in diabetic individuals induces accumulation of the highly reactive dicarbonyl compound methylglyoxal (MGO), which modulates TRPA1 activity. Long-term oral intake of MGO causes mouse bladder dysfunction. We hypothesized that TRPA1 takes part in the machinery that leads to MGO-induced bladder dysfunction. Therefore, we evaluated TRPA1 expression in the bladder and the effects of 1 h-intravesical infusion of the selective TRPA1 blocker HC-030031 (1 nmol/min) on MGO-induced cystometric alterations. Methods: Five-week-old female C57BL/6 mice received 0.5% MGO in their drinking water for 12 weeks, whereas control mice received tap water alone. Results: Compared to the control group, the protein levels and immunostaining for the MGO-derived hydroimidazolone isomer MG-H1 was increased in bladders of the MGO group, as observed in urothelium and detrusor smooth muscle. TRPA1 protein expression was significantly higher in bladder tissues of MGO compared to control group with TRPA1 immunostaining both lamina propria and urothelium, but not the detrusor smooth muscle. Void spot assays in conscious mice revealed an overactive bladder phenotype in MGO-treated mice characterized by increased number of voids and reduced volume per void. Filling cystometry in anaesthetized animals revealed an increased voiding frequency, reduced bladder capacity, and reduced voided volume in MGO compared to vehicle group, which were all reversed by HC-030031 infusion. Conclusion: TRPA1 activation is implicated in MGO-induced mouse overactive bladder. TRPA1 blockers may be useful to treat diabetic bladder dysfunction in individuals with high MGO levels.
Keywords: MG-H1; cystometry; glyoxalase; lamina propria; urothelium; void spot assay.
Copyright © 2023 Oliveira, Medeiros, Gomes, Mello, Costa, Mónica and Antunes.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Abrams P., Andersson K. E., Buccafusco J. J., Chapple C., de Groat W. C., Fryer A. D., et al. (2006). Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br. J. Pharmacol. 148 (5), 565–578. 10.1038/sj.bjp.0706780 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

