Trends in SARS-CoV-2 seroprevalence in Albania during the 2021-2022 pandemic year
- PMID: 38143941
- PMCID: PMC10746500
- DOI: 10.1016/j.nmni.2023.101208
Trends in SARS-CoV-2 seroprevalence in Albania during the 2021-2022 pandemic year
Abstract
Background: Monitoring SARS-CoV-2 seroprevalence dynamics during the COVID-19 pandemic is crucial for understanding population immunity and providing insights into public health policies. Limited data exist on this from Albania and other Eastern European countries. This study aimed to investigate SARS-CoV-2 seroprevalence in Albania, comparing August 2021 and August 2022 data from two representative samples of the general population. The objective was to understand the temporal dynamics of SARS-CoV-2 antibodies across age groups and assess the impacts of natural infection and vaccination on population immunity.
Methods: This longitudinal study was conducted in two consecutive cross-sectional assessments 12 months apart in Albania's urban all-ages population. IgG anti-Spike-1 and anti-Nucleoprotein SARS-CoV-2 antibodies were measured using ELISA, focusing on seropositivity rates and antibody levels.
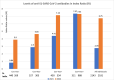
Methods: The study encompassed 2143 and 2183 individuals in August 2021 and 2022, respectively, with the anti-S1-IgG seropositivity rate escalating from 70.9 % to 92.1 %. In 2021, seroprevalence ranged from 49.6 % (0-15 years) to 82 % (>60 years). By August 2022, it surpassed 90 % in most age groups, except 0-15 years (73.8 %). "Hybrid" immunity (COVID-19+ and Vaccine+) reached 56.6 % in 2022, or 2.8 times higher than in 2021, exhibiting the highest antibody levels compared to the only vaccinated or previously COVID-19-infected individuals.
Conclusion: This study highlights an overall 94 % seroprevalence in the Albanian population in August 2022 and robust "hybrid" immunity, suggesting substantial protective immunity against SARS-CoV-2. The lower immunity in the 0-15 age group underscores the necessity for youth-targeted vaccine campaigns. These findings provide valuable insights for shaping healthcare measures and vaccination policies.
Keywords: Albania; COVID-19; Eastern Europe; SARS-CoV-2; SARS-CoV-2 IgG antibodies; Seroprevalence.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Figueiredo-Campos P., Blankenhaus B., Mota C., Gomes A., Serrano M., Ariotti S., et al. Seroprevalence of anti-SARS-CoV-2 antibodies in COVID-19 patients and healthy volunteers up to 6 months post disease onset. Eur J Immunol. 2020 Dec;50(12):2025–2040. doi: 10.1002/eji.202048970. Epub 2020 Nov 10. PMID: 33084029; PMCID: PMC7756220. - DOI - PMC - PubMed
-
- Feng S., Phillips D.J., White T., Sayal H., Aley P.K., Bibi S., et al. Oxford COVID Vaccine Trial Group. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021 Nov;27(11):2032–2040. doi: 10.1038/s41591-021-01540-1. Epub 2021 Sep 29. PMID: 34588689; PMCID: PMC8604724. - DOI - PMC - PubMed
-
- Earle K.A., Ambrosino D.M., Fiore-Gartland A., Goldblatt D., Gilbert P.B., Siber G.R., et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021 Jul 22;39(32):4423–4428. doi: 10.1016/j.vaccine.2021.05.063. Epub 2021 May 24. PMID: 34210573; PMCID: PMC8142841. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous