Study of Drug Delivery Using Purely Organic Macrocyclic Containers-Cucurbit[7]uril and Pillararene
- PMID: 38144095
- PMCID: PMC10733925
- DOI: 10.1021/acsomega.3c05465
Study of Drug Delivery Using Purely Organic Macrocyclic Containers-Cucurbit[7]uril and Pillararene
Abstract
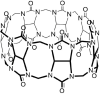
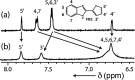
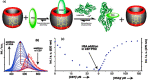
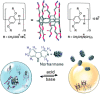
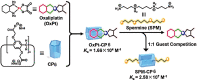
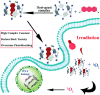
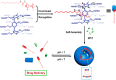
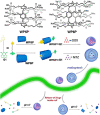
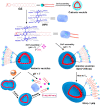
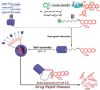
An impaired immune system is the root of various human ailments provoking the urge to find vehicle-mediated quick delivery of small drug molecules and other vital metabolites to specific tissues and organs. Thus, drug delivery strategies are in need of improvement in therapeutic efficacy. It can be achieved only by increasing the drug-loading capacity, increasing the sustained release of a drug to its target site, easy relocation of drug molecules associated with facile complexation-induced properties of molecular vehicles, and high stimuli-responsive drug administration. Supramolecular drug delivery systems (SDDS) provide a much needed robust yet facile platform for fabricating innovative drug nanocarriers assembled by thermodynamically noncovalent interaction with the tunable framework and above-mentioned properties. Measures of cytotoxicity and biocompatibility are the two main criteria that lie at the root of any promising medicinal applications. This Review features significant advancements in (i) supramolecular host-guest complexation using cucurbit[7]uril (CB[7]), (ii) encapsulation of the drug and its delivery application tailored for CB[7], (iii) self-assembly of supramolecular amphiphiles, (iv) supramolecular guest relay using host-protein nanocavities, (v) pillararene (a unique macrocyclic host)-mediated SDDS for the delivery of smart nanodrugs for siRNA, fluorescent molecules, and insulin for juvenile diabetes. Furthermore, fundamental questions and future hurdles related to smart SDDS based on CB[7] and pillararenes and their future promising breakthrough implementations are also distinctly outlined in this Review.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
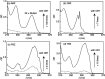

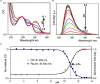
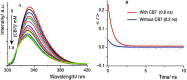
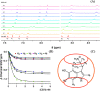

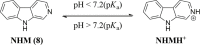
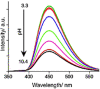
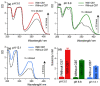
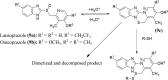
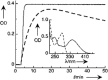


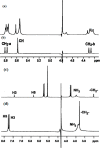
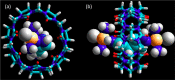
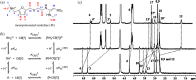
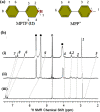
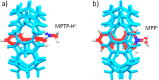
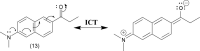

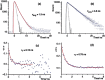



References
-
- Da Silva E.; Lazar A.; Coleman A. Biopharmaceutical applications of calixarenes. J. Drug Deliv Sci. Technol. 2004, 14, 3–20. 10.1016/S1773-2247(04)50001-1. - DOI
-
- Gillan R.Modelling molecules for drug delivery; Royal Society of Chemistry, Thomas Graham House: Science Park, Milton Road, Cambridge, 2006.
Publication types
LinkOut - more resources
Full Text Sources

