Insights on the Synthesis of Al-MCM-41 with Optimized Si/Al Ratio for High-Performance Antibiotic Adsorption
- PMID: 38144102
- PMCID: PMC10733947
- DOI: 10.1021/acsomega.3c07119
Insights on the Synthesis of Al-MCM-41 with Optimized Si/Al Ratio for High-Performance Antibiotic Adsorption
Abstract
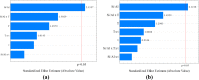
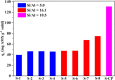
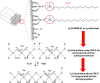
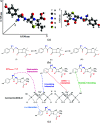
Studies indicate that approximately two-thirds of the rivers of the world are contaminated by pharmaceutical compounds, especially antibiotics and hormones. Data reported by the World Health Organization (WHO, 2015) revealed an increase of 65% in antibiotic consumption between 2000 and 2015, with a worldwide increase of 200% expected up to 2030. Environmental contamination by antibiotics and their metabolites can cause the alteration of bacterial genes, leading to the generation of superbacteria. In this work, adsorption was explored as a strategy to mitigate antibiotic contamination, proposing the use of the Al-MCM-41 mesoporous material as an efficient and high-capacity adsorbent. Evaluation of the influence of the synthesis parameters enabled understanding of the main variables affecting the adsorption capacity of Al-MCM-41 for the removal of a typical antibiotic, amoxicillin (AMX). It was found that the adsorbent composition and specific surface area were the main factors that should be optimized in order to obtain the highest AMX removal capacity. Using statistical tools, the best Si/Al ratio in Al-MCM-41 was found to be 10.5, providing an excellent AMX uptake of 132.2 mg per gram of adsorbent. The Si/Al ratio was the most significant factor affecting the adsorption. The cation-π interactions increased with an increase of the Al content, while the interactions involving silanols (Yoshida H-bonding and dipole-dipole hydrogen bridges) decreased.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Yazidi A.; Atrous M.; Edi Soetaredjo F.; Sellaoui L.; Ismadji S.; Erto A.; Bonilla-Petriciolet A.; Luiz Dotto G.; Ben Lamine A. Adsorption of amoxicillin and tetracycline on activated carbon prepared from durian shell in single and binary systems: Experimental study and modeling analysis. Chem. Eng. J. 2020, 379, 12232010.1016/j.cej.2019.122320. - DOI
-
- Imanipoor J.; Ghafelebashi A.; Mohammadi M.; Dinari M.; Ehsani M. R. Fast and effective adsorption of amoxicillin from aqueous solutions by L-methionine modified montmorillonite K10. Colloids Surf., A 2021, 611, 12579210.1016/j.colsurfa.2020.125792. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

