A novel animal model of neuropathic corneal pain-the ciliary nerve constriction model
- PMID: 38144209
- PMCID: PMC10749205
- DOI: 10.3389/fnins.2023.1265708
A novel animal model of neuropathic corneal pain-the ciliary nerve constriction model
Abstract
Introduction: Neuropathic pain arises as a result of peripheral nerve injury or altered pain processing within the central nervous system. When this phenomenon affects the cornea, it is referred to as neuropathic corneal pain (NCP), resulting in pain, hyperalgesia, burning, and photoallodynia, severely affecting patients' quality of life. To date there is no suitable animal model for the study of NCP. Herein, we developed an NCP model by constriction of the long ciliary nerves innervating the eye.
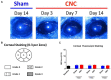
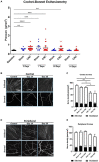
Methods: Mice underwent ciliary nerve constriction (CNC) or sham procedures. Safety was determined by corneal fluorescein staining to assess ocular surface damage, whereas Cochet-Bonnet esthesiometry and confocal microscopy assessed the function and structure of corneal nerves, respectively. Efficacy was assessed by paw wipe responses within 30 seconds of applying hyperosmolar (5M) saline at Days 3, 7, 10, and 14 post-constriction. Additionally, behavior was assessed in an open field test (OFT) at Days 7, 14, and 21.
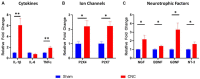
Results: CNC resulted in significantly increased response to hyperosmolar saline between groups (p < 0.0001), demonstrating hyperalgesia and induction of neuropathic pain. Further, animals that underwent CNC had increased anxiety-like behavior in an open field test compared to controls at the 14- and 21-Day time-points (p < 0.05). In contrast, CNC did not result in increased corneal fluorescein staining or decreased sensation as compared to sham controls (p > 0.05). Additionally, confocal microscopy of corneal whole-mounts revealed that constriction resulted in only a slight reduction in corneal nerve density (p < 0.05), compared to naïve and sham groups.
Discussion: The CNC model induces a pure NCP phenotype and may be a useful model for the study of NCP, recapitulating features of NCP, including hyperalgesia in the absence of ocular surface damage, and anxiety-like behavior.
Keywords: animal model; chronic constriction injury; neuropathic corneal pain; nociception; ocular pain.
Copyright © 2023 Seyed-Razavi, Kenyon, Qiu, Harris and Hamrah.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



Similar articles
-
Clinical and in vivo confocal microscopic features of neuropathic corneal pain.Br J Ophthalmol. 2020 Jun;104(6):768-775. doi: 10.1136/bjophthalmol-2019-314799. Epub 2019 Sep 18. Br J Ophthalmol. 2020. PMID: 31533927
-
Visualization of microneuromas by using in vivo confocal microscopy: An objective biomarker for the diagnosis of neuropathic corneal pain?Ocul Surf. 2020 Oct;18(4):651-656. doi: 10.1016/j.jtos.2020.07.004. Epub 2020 Jul 11. Ocul Surf. 2020. PMID: 32663518 Free PMC article.
-
Efficacy of autologous serum tears for treatment of neuropathic corneal pain.Ocul Surf. 2019 Jul;17(3):532-539. doi: 10.1016/j.jtos.2019.01.009. Epub 2019 Jan 24. Ocul Surf. 2019. PMID: 30685437 Free PMC article.
-
Neuropathic corneal pain and dry eye: a continuum of nociception.Br J Ophthalmol. 2022 Aug;106(8):1039-1043. doi: 10.1136/bjophthalmol-2020-318469. Epub 2021 Apr 30. Br J Ophthalmol. 2022. PMID: 33931393 Review.
-
[Research progress of diagnosis in neuropathic corneal pain].Zhonghua Yan Ke Za Zhi. 2022 Aug 11;58(8):629-634. doi: 10.3760/cma.j.cn112142-20211110-00531. Zhonghua Yan Ke Za Zhi. 2022. PMID: 35959608 Review. Chinese.
Cited by
-
Modeling Neuropathic Corneal Pain: Pulled Nerve Approach With Elevated Krt16 Gene Expression.Invest Ophthalmol Vis Sci. 2025 Feb 3;66(2):35. doi: 10.1167/iovs.66.2.35. Invest Ophthalmol Vis Sci. 2025. PMID: 39937496 Free PMC article.
-
Evaluation of Corneal Sensitivity: Tools We Have.Diagnostics (Basel). 2025 Jul 15;15(14):1785. doi: 10.3390/diagnostics15141785. Diagnostics (Basel). 2025. PMID: 40722534 Free PMC article. Review.
References
-
- Aulehner K., Leenaars C., Buchecker V., Stirling H., Schonhoff K., King H., et al. . (2022). Grimace scale, burrowing, and nest building for the assessment of post-surgical pain in mice and rats-a systematic review. Front. Vet. Sci. 9:930005. doi: 10.3389/fvets.2022.930005, PMID: - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

