Alternative immune checkpoints in immunoregulatory profile of cancer stem cells
- PMID: 38144305
- PMCID: PMC10746460
- DOI: 10.1016/j.heliyon.2023.e23171
Alternative immune checkpoints in immunoregulatory profile of cancer stem cells
Abstract
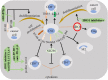
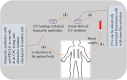
Tumor-mediated bypass of immune checkpoint inhibitor (ICI) therapy with anti-programmed death-1 (PD-1), anti-programmed death-ligand 1 (PD-L1, also called B7-H1 or CD274) or anti-cytotoxic T lymphocyte associated antigen-4 (CTLA-4) is a challenge of current years in the area of cancer immunotherapy. Alternative immune checkpoints (AICs) are molecules beyond the common PD-1, PD-L1 or CTLA-4, and are upregulated in patients who show low/no ICI responses. These are members of B7 family including B7-H2 (ICOS-L), B7-H3 (CD276), B7-H4 (B7x), V-domain immunoglobulin suppressor of T cell activation (VISTA), B7-H6, HHLA2 (B7-H5/B7-H7) and catabolic enzymes like indoleamine 2,3-dioxygenase 1 (IDO1), and others that are also contributed to the regulation of tumor immune microenvironment (TIME). There is also strong evidence supporting the implication of AICs in regulation of cancer stemness and expanding the population of cancer stem cells (CSCs). CSCs display immunoregulatory capacity and represent multiple immune checkpoints either on their surface or inside. Besides, they are active promoters of resistance to the common ICIs. The aim of this review is to investigate interrelations between AICs with stemness and differentiation profile of cancer. The key message of this paper is that targeted checkpoints can be selected based on their impact on CSCs along with their effect on immune cells. Studies published so far mainly focused on immune cells as a target for anti-checkpoints. Ex vivo engineering of extracellular vesicles (EVs) equipped with CSC-targeted anti-checkpoint antibodies is without a doubt a key therapeutic target that can be under consideration in future research.
Keywords: Alternative immune checkpoint (AIC); Cancer stem cell (CSC); Immune checkpoint inhibitor (ICI); Resistance; Tumor microenvironment (TME).
© 2023 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The manuscript entitled ‘Alternative immune checkpoints in immunoregulatory profile of cancer stem cells’ is the representative of novel insights toward the impact of alternative immune checkpoints in cancer stemness profile and using the strategy in improving cancer immunotherapy outcomes. All figures are originally stated and the idea is novel, and both authors are contributed significantly to the subject.
Figures
References
-
- Rouzbahani E., Majidpoor J., Najafi S., Mortezaee K. Cancer stem cells in immunoregulation and bypassing anti-checkpoint therapy. Biomed. Pharmacother. 2022;156 - PubMed
-
- Najafi S., Majidpoor J., Mortezaee K. Life Sciences; 2022. The Impact of Microbiota on PD-1/pd-L1 Inhibitor Therapy Outcomes: A Focus on Solid Tumors. - PubMed
-
- Mortezaee K. Redox tolerance and metabolic reprogramming in solid tumors. Cell Biol. Int. 2021;45(2):273–286. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

