Mice prone to tinnitus after acoustic trauma show increased pre-exposure sensitivity to background noise
- PMID: 38144362
- PMCID: PMC10739389
- DOI: 10.3389/fnbeh.2023.1321277
Mice prone to tinnitus after acoustic trauma show increased pre-exposure sensitivity to background noise
Abstract
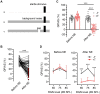
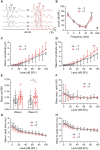
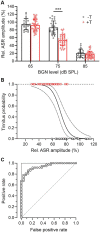
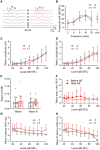
Noise-induced tinnitus is generally associated with hearing impairment caused by traumatic acoustic overexposure. Previous studies in laboratory animals and human subjects, however, have observed differences in tinnitus susceptibility, even among individuals with similar hearing loss. The mechanisms underlying increased sensitivity or, conversely, resistance to tinnitus are still incompletely understood. Here, we used behavioral tests and ABR audiometry to compare the sound-evoked responses of mice that differed in the presence of noise-induced tinnitus. The aim was to find a specific pre-exposure neurophysiological marker that would predict the development of tinnitus after acoustic trauma. Noise-exposed mice were screened for tinnitus-like behavior with the GPIAS paradigm and subsequently divided into tinnitus (+T) and non-tinnitus (-T) groups. Both groups showed hearing loss after exposure, manifested by elevated audiometric thresholds along with reduced amplitudes and prolonged latencies of ABR waves. Prior to exposure, except for a slightly increased slope of growth function for ABR amplitudes in +T mice, the two groups did not show significant audiometric differences. Behavioral measures, such as the magnitude of the acoustic startle response and its inhibition by gap pre-pulse, were also similar before exposure in both groups. However, +T mice showed significantly increased suppression of the acoustic startle response in the presence of background noise of moderate intensity. Thus, increased modulation of startle by background sounds may represent a behavioral correlate of susceptibility to noise-induced tinnitus, and its measurement may form the basis of a simple non-invasive method for predicting tinnitus development in laboratory rodents.
Keywords: acoustic startle reflex; background noise; hearing loss; noise exposure; tinnitus.
Copyright © 2023 Rybalko, Suchánková, Bureš, Jovanović, Melichar, Profant and Tureček.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

