GIPC1 regulates MACC1-driven metastasis
- PMID: 38144523
- PMCID: PMC10748395
- DOI: 10.3389/fonc.2023.1280977
GIPC1 regulates MACC1-driven metastasis
Abstract
Background: Identification of cancer metastasis-relevant molecular networks is desired to provide the basis for understanding and developing intervention strategies. Here we address the role of GIPC1 in the process of MACC1-driven metastasis. MACC1 is a prognostic indicator for patient metastasis formation and metastasis-free survival. MACC1 controls gene transcription, promotes motility, invasion and proliferation of colon cancer cells in vitro, and causes tumor growth and metastasis in mice.
Methods: By using yeast-two-hybrid assay, mass spectrometry, co-immunoprecipitation and peptide array we analyzed GIPC1 protein binding partners, by using the MACC1 gene promoter and chromatin immunoprecipitation and electrophoretic mobility shift assay we probed for GIPC1 as transcription factor. We employed GIPC1/MACC1-manipulated cell lines for in vitro and in vivo analyses, and we probed the GIPC1/MACC1 impact using human primary colorectal cancer (CRC) tissue.
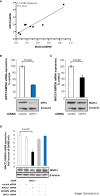
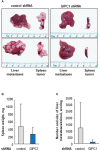
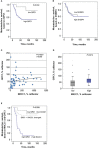
Results: We identified MACC1 and its paralogue SH3BP4 as protein binding partners of the protein GIPC1, and we also demonstrated the binding of GIPC1 as transcription factor to the MACC1 promoter (TSS to -60 bp). GIPC1 knockdown reduced endogenous, but not CMV promoter-driven MACC1 expression, and diminished MACC1-induced cell migration and invasion. GIPC1 suppression reduced tumor growth and metastasis in mice intrasplenically transplanted with MACC1-overexpressing CRC cells. In human primary CRC specimens, GIPC1 correlates with MACC1 expression and is of prognostic value for metastasis formation and metastasis-free survival. Combination of MACC1 and GIPC1 expression improved patient survival prognosis, whereas SH3BP4 expression did not show any prognostic value.
Conclusions: We identified an important, dual function of GIPC1 - as protein interaction partner and as transcription factor of MACC1 - for tumor progression and cancer metastasis.
Keywords: GIPC1; MACC1; patient survival prognosis; protein-protein interaction; transcription factor.
Copyright © 2023 Siegel, Schmidt, Juneja, Smith, Herrmann, Kobelt, Sharma, Fichtner, Walther, Dittmar, Volkmer, Rathjen, Schlag and Stein.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
LinkOut - more resources
Full Text Sources

