Hepatocyte-targeted siTAZ therapy lowers liver fibrosis in NASH diet-fed chimeric mice with hepatocyte-humanized livers
- PMID: 38144682
- PMCID: PMC10746533
- DOI: 10.1016/j.omtm.2023.101165
Hepatocyte-targeted siTAZ therapy lowers liver fibrosis in NASH diet-fed chimeric mice with hepatocyte-humanized livers
Abstract
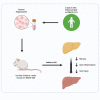
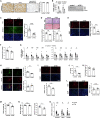
Nonalcoholic steatohepatitis (NASH) is emerging as the most common cause of liver disease. Although many studies in mouse NASH models have suggested therapies, translation to humans is poor, with no approved drugs for NASH. One explanation may lie in differences between mouse and human hepatocytes. We used NASH diet-fed chimeric mice reconstituted with human hepatocytes (hu-liver mice) to test a mechanism-based hepatocyte-targeted small interfering RNA (siRNA), GalNAc-siTaz, shown previously to block the progression to fibrotic NASH in mice. Following ablation of endogenous hepatocytes, male mice were reconstituted with human hepatocytes from a single donor with the rs738409-C/G PNPLA3 risk variant, resulting in ∼95% human hepatocyte reconstitution. The mice were then fed a high-fat choline-deficient l-amino acid-defined diet for 6 weeks to induce NASH, followed by six weekly injections of GalNAc-siTAZ to silence hepatocyte-TAZ or control GalNAc-siRNA (GalNAc-control) while still on the NASH diet. GalNAc-siTAZ lowered human hepatic TAZ and IHH, a TAZ target that promotes NASH fibrosis. Most important, GalNAc-siTAZ decreased liver inflammation, hepatocellular injury, hepatic fibrosis, and profibrogenic mediator expression versus GalNAc-control, indicating that GalNAc-siTAZ decreased the progression of NASH in mice reconstituted with human hepatocytes. In conclusion, silencing TAZ in human hepatocytes suppresses liver fibrosis in a hu-liver model of NASH.
Keywords: GalNAc-siRNA; IHH; MASH; NASH/MASH therapy, NASH, TAZ, Indian hedgehog; WWTR1; hepatocyte-humanized mice; liver fibrosis.
© 2023 The Author(s).
Conflict of interest statement
The laboratory of I.T. received research funding, material support, and technical input from the Takeda Pharmaceutical Company to study siTAZ therapeutics, for which Columbia University holds a patent.
Figures

References
-
- Harrison S.A., Allen A.M., Dubourg J., Noureddin M., Alkhouri N. Challenges and opportunities in NASH drug development. Nat. Med. 2023;29:562–573. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

