Estimating SARS-CoV-2 seroprevalence
- PMID: 38145241
- PMCID: PMC10746549
- DOI: 10.1093/jrsssa/qnad068
Estimating SARS-CoV-2 seroprevalence
Abstract
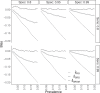
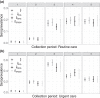
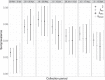
Governments and public health authorities use seroprevalence studies to guide responses to the COVID-19 pandemic. Seroprevalence surveys estimate the proportion of individuals who have detectable SARS-CoV-2 antibodies. However, serologic assays are prone to misclassification error, and non-probability sampling may induce selection bias. In this paper, non-parametric and parametric seroprevalence estimators are considered that address both challenges by leveraging validation data and assuming equal probabilities of sample inclusion within covariate-defined strata. Both estimators are shown to be consistent and asymptotically normal, and consistent variance estimators are derived. Simulation studies are presented comparing the estimators over a range of scenarios. The methods are used to estimate severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) seroprevalence in New York City, Belgium, and North Carolina.
Keywords: COVID-19; diagnostic tests; estimating equations; seroepidemiologic studies; standardization.
© The Royal Statistical Society 2023. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest There is no conflict of interest.
Figures


References
-
- Accorsi E. K., Qiu X., Rumpler E., Kennedy-Shaffer L., Kahn R., Joshi K., Goldstein E., Stensrud M. J., Niehus R., Cevik M., & Lipsitch M. (2021). How to detect and reduce potential sources of biases in studies of SARS-CoV-2 and COVID-19. European Journal of Epidemiology, 36(2), 179–196. 10.1007/s10654-021-00727-7 - DOI - PMC - PubMed
-
- Arora R. K., Joseph A., Van Wyk J., Rocco S., Atmaja A., May E., Yan T., Bobrovitz N., Chevrier J., Cheng M. P., Williamson T., & Buckeridge D. L. (2021). SeroTracker: A global SARS-CoV-2 seroprevalence dashboard. The Lancet Infectious Diseases, 21(4), e75–e76. 10.1016/S1473-3099(20)30631-9 - DOI - PMC - PubMed
-
- Bajema K. L., Wiegand R. E., Cuffe K., Patel S. V., Iachan R., Lim T., Lee A., Moyse D., Havers F. P., Harding L., Fry A. M., Hall A. J., Martin K., Biel M., Deng Y., MeyerW. A., III., Mathur M., Kyle T., Gundlapalli A. V., … Edens C. (2021). Estimated SARS-CoV-2 seroprevalence in the US as of September 2020. JAMA Internal Medicine, 181(4), 450–460. 10.1001/jamainternmed.2020.7976 - DOI - PMC - PubMed
-
- Barzin A., Schmitz J. L., Rosin S., Sirpal R., Almond M., Robinette C., Wells S., Hudgens M., Olshan A., Deen S., Krejci P., Quackenbush E., Chronowski K., Cornaby C., Goins J., Butler L., Aucoin J., Boyer K., Faulk J., … Peden D. B. (2020). SARS-CoV-2 seroprevalences among a southern U.S. population indicates limited asymptomatic spread under physical distancing measures. mBio, 11(5), e02426-20. 10.1128/mBio.02426-20 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
