Cenicriviroc prevents dysregulation of astrocyte/endothelial cross talk induced by ischemia and HIV-1 via inhibiting the NLRP3 inflammasome and pyroptosis
- PMID: 38145295
- PMCID: PMC11192487
- DOI: 10.1152/ajpcell.00600.2023
Cenicriviroc prevents dysregulation of astrocyte/endothelial cross talk induced by ischemia and HIV-1 via inhibiting the NLRP3 inflammasome and pyroptosis
Abstract
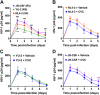
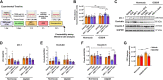
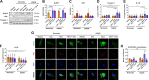
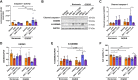
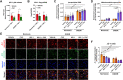
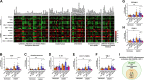
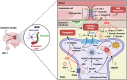
Blood-brain barrier (BBB) breakdown is one of the pathophysiological characteristics of ischemic stroke, which may contribute to the progression of brain tissue damage and subsequent neurological impairment. Human immunodeficiency virus (HIV)-infected individuals are at greater risk for ischemic stroke due to diminished immune function and HIV-associated vasculopathy. Studies have shown that astrocytes are involved in maintaining BBB integrity and facilitating HIV-1 infection in the brain. The present study investigated whether targeting astrocyte-endothelial cell signaling with cenicriviroc (CVC), a dual chemokine receptor (CCR)2 and CCR5 antagonist, may protect against dysregulation of cross talk between these cells after oxygen-glucose deprivation/reoxygenation (OGD/R) combined with HIV-1 infection. Permeability assay with 10 kDa fluorescein isothiocyanate (FITC)-dextran demonstrated that CVC alleviated endothelial barrier disruption in noncontact coculture of human brain microvascular endothelial cells (HBMECs) with HIV-1-infected human astrocytes, and reversed downregulation of tight junction protein claudin-5 induced by OGD/R- and HIV-1. Moreover, CVC attenuated OGD/R- and HIV-1-triggered upregulation of the NOD-like receptor protein-3 (NLRP3) inflammasome and IL-1β secretion. Treatment with CVC also suppressed astrocyte pyroptosis by attenuating cleaved caspase-1 levels and the formation of cleaved N-terminal GSDMD (N-GSDMD). Secretome profiling revealed that CVC ameliorated secretion levels of chemokine CC chemokine ligand 17 (CCL17), adhesion molecule intercellular adhesion molecule-1 (ICAM-1), and T cell activation modulator T cell immunoglobulin and mucin domain 3 (TIM-3) by astrocytes synergistically induced by OGD/R and HIV-1. Overall, these results suggest that CVC contributes to restoring astrocyte-endothelial cross interactions in an astrocyte-dependent manner via protection against NLRP3 activation and pyroptosis.NEW & NOTEWORTHY The present study reveals the role of astrocytic NOD-like receptor protein-3 (NLRP3) inflammasome in dysfunctional astrocyte-endothelial cross interactions triggered in response to oxygen/glucose deprivation injury associated with human immunodeficiency virus type 1 (HIV-1) infection. Our results suggest that blocking NLRP3 inflammasome activation and pyroptosis-mediated inflammation with cenicriviroc (CVC) may constitute a potentially effective therapeutic strategy for blood-brain barrier (BBB) protection during HIV-1-associated ischemic stroke.
Keywords: HIV-1; NLRP3 inflammasome; blood-brain barrier; ischemia and reperfusion; ischemic stroke.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

Similar articles
-
The role of NLRP3 inflammasome-mediated pyroptosis in astrocytes during hyperoxia-induced brain injury.Inflamm Res. 2025 Jan 25;74(1):25. doi: 10.1007/s00011-024-01984-4. Inflamm Res. 2025. PMID: 39862240 Review.
-
Lipocalin-2-mediated astrocyte pyroptosis promotes neuroinflammatory injury via NLRP3 inflammasome activation in cerebral ischemia/reperfusion injury.J Neuroinflammation. 2023 Jun 23;20(1):148. doi: 10.1186/s12974-023-02819-5. J Neuroinflammation. 2023. PMID: 37353794 Free PMC article.
-
NLPR3 inflammasome inhibition alleviates hypoxic endothelial cell death in vitro and protects blood-brain barrier integrity in murine stroke.Cell Death Dis. 2021 Dec 20;13(1):20. doi: 10.1038/s41419-021-04379-z. Cell Death Dis. 2021. PMID: 34930895 Free PMC article.
-
Fine particulate matter exposure aggravates ischemic injury via NLRP3 inflammasome activation and pyroptosis.CNS Neurosci Ther. 2022 Jul;28(7):1045-1058. doi: 10.1111/cns.13837. Epub 2022 Apr 10. CNS Neurosci Ther. 2022. PMID: 35403328 Free PMC article.
-
[Research progress on the pyroptosis mediated by the NLRP3 inflammasome in the pathology of ischemic stroke].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2024 Jul;40(7):648-654. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2024. PMID: 39179409 Review. Chinese.
Cited by
-
Induced neural stem cells ameliorate blood-brain barrier injury by modulating the calcium signaling pathway of astrocyte in cerebral ischemia-reperfusion rats.Front Cell Dev Biol. 2025 Jun 3;13:1611226. doi: 10.3389/fcell.2025.1611226. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40530334 Free PMC article.
-
Methamphetamine and HIV-1 Tat Synergistically Induce Microglial Pyroptosis Via Activation of the AIM2 Inflammasome.Inflammation. 2025 Feb 19. doi: 10.1007/s10753-025-02266-9. Online ahead of print. Inflammation. 2025. PMID: 39969742
-
New Therapies and Strategies to Curb HIV Infections with a Focus on Macrophages and Reservoirs.Viruses. 2024 Sep 18;16(9):1484. doi: 10.3390/v16091484. Viruses. 2024. PMID: 39339960 Free PMC article. Review.
-
Huanglian Jiedu Decoction Treats Ischemic Stroke by Regulating Pyroptosis: Insights from Multi-Omics and Drug-Target Relationship Analysis.Pharmaceuticals (Basel). 2025 May 23;18(6):775. doi: 10.3390/ph18060775. Pharmaceuticals (Basel). 2025. PMID: 40573172 Free PMC article.
-
The role of NLRP3 inflammasome-mediated pyroptosis in astrocytes during hyperoxia-induced brain injury.Inflamm Res. 2025 Jan 25;74(1):25. doi: 10.1007/s00011-024-01984-4. Inflamm Res. 2025. PMID: 39862240 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

