Organic Donor-Acceptor Systems for Photocatalysis
- PMID: 38145342
- PMCID: PMC10933655
- DOI: 10.1002/advs.202307227
Organic Donor-Acceptor Systems for Photocatalysis
Abstract
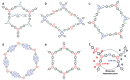
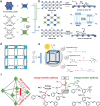
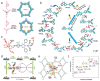
Organic semiconductor materials are considered to be promising photocatalysts due to their excellent light absorption by chromophores, easy molecular structure tuning, and solution-processable properties. In particular, donor-acceptor (D-A) type organic photocatalytic materials synthesized by introducing D and A units intra- or intermolecularly, have made great progress in photocatalytic studies. More and more studies have demonstrated that the D-A type organic photocatalytic materials combine effective carrier separation, tunable bandgap, and sensitive optoelectronic response, and are considered to be an effective strategy for enhancing light absorption, improving exciton dissociation, and optimizing carrier transport. This review provides a thorough overview of D-A strategies aimed at optimizing the photocatalytic performance of organic semiconductors. Initially, essential methods for modifying organic photocatalytic materials, such as interface engineering, crystal engineering, and interaction modulation, are briefly discussed. Subsequently, the review delves into various organic photocatalytic materials based on intramolecular and intermolecular D-A interactions, encompassing small molecules, conjugated polymers, crystalline polymers, supramolecules, and organic heterojunctions. Meanwhile, the energy band structures, exciton dynamics, and redox-active sites of D-A type organic photocatalytic materials under different bonding modes are discussed. Finally, the review highlights the advanced applications of organic photocatalystsand outlines prospective challenges and opportunities.
Keywords: D-A interactions; exciton dissociation; organic semiconductors; photocatalysis.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


















References
-
- Miao Y., Zhao Y., Zhang S., Shi R., Zhang T., Adv. Mater. 2022, 34, 2200868. - PubMed
-
- He T., Zhao Y., Angew. Chem., Int. Ed. 2023, 62, 202303086.
-
- Sun X., Huang H., Zhao Q., Ma T., Wang L., Adv. Funct. Mater. 2020, 30, 1910005.
-
- He J., Liu Y., Qu J., Xie H., Lu R., Fan F., Li C., Adv. Mater. 2023, 35, 2210374. - PubMed
-
- Gong E., Ali S., Hiragond C. B., Kim H. S., Powar N. S., Kim D., Kim H., In S.‐I., Energy Environ. Sci. 2022, 15, 880.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
